“The lower the better” is an important principle for lipid-lowering therapies, and PCSK9 monoclonal antibodies (PCSK9 mAb), such as evolocumab, can effectively lower LDL-C levels [1]. Data from the ECARA and HUYGENS studies presented at the European Society of Cardiology Annual Congress 2021 shed more light on LDL-C lowering by evolocumab under different conditions [2,3].
Elevated LDL-C levels are directly associated with the development of atherosclerotic plaques and the risk of cardiovascular events [4]. The current recommendations of the ESC/EAS as well as the AGLA recommend a LDL-C reduction of ≥50% and a LDL-C target of <1.4 mmol/L in cardiovascular high-risk patients, which, however, is not always achieved in practice [5,6]. At this year’s ESC Congress, recent results from the Swiss observational study ECARA and the randomized phase III HUYGENS trial were presented, highlighting the relevance of early use of the PCSK9 mAb Evolocumab (Repatha®) [2,3].
ECARA: High adherence and efficacy of evolocumab in real-world settings
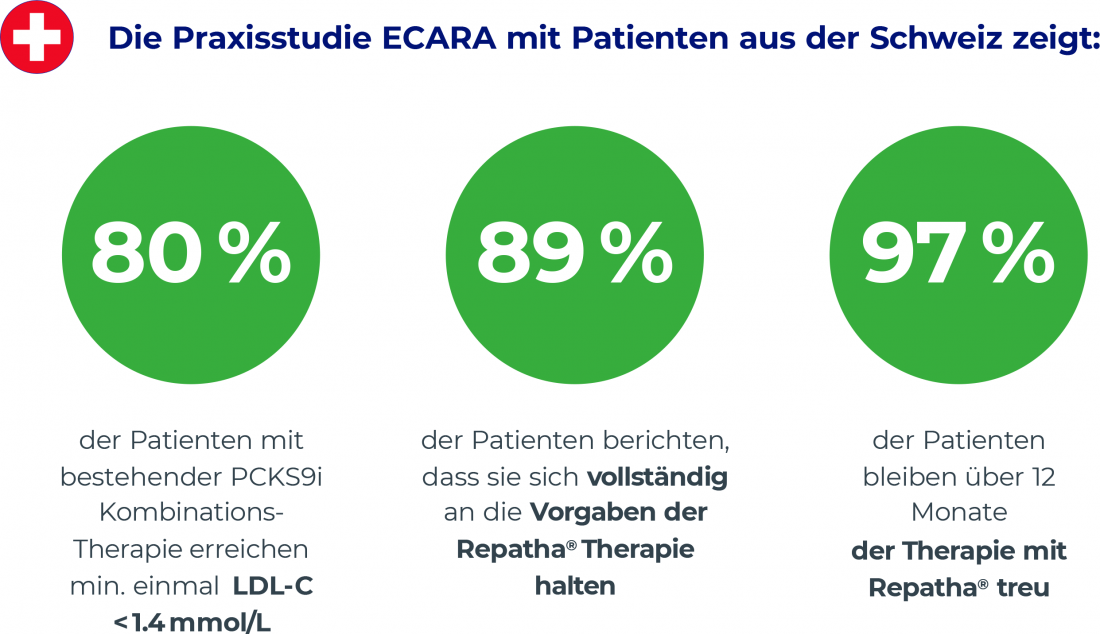
The ECARA study provides evidence for LDL-C lowering by evolocumab in cardiovascular risk patients in Swiss daily practice [2]. The multicenter observational study examined adults (median age: 61 years) with confirmed atherosclerotic cardivascular disease or high cardiovascular risk and elevated LDL-C levels despite maximally tolerated statin therapy. 81% of participants had at least one prior cardiovascular event at baseline, 55% had two or more. The efficacy of evolocumab was compared in patients with and without PCSK9 mAb pretreatment. The median baseline LDL-C was 1.9 mmol/L, and LDL-C levels at 3, 6, and 12 months served as the primary study end point [2]. Overall, treatment proved effective under real-world conditions, with 69% of PCSK9 mAb-naive and 68% of PCSK9 mAb-pretreated patients achieving the LDL-C target of <1.4 mmol/L at least once. The results also indicate the beneficial efficacy of combining evolocumab with other lipid-lowering therapies. Thus, with combination therapy, 74% of PCSK9 mAb-naive patients achieved an LDL-C level of <1.4 mmol/L, compared with 80% of PCSK9 mAb-pretreated patients [3]. Regarding adherence, 89% of participants reported complete adherence to planned evolocumab therapy (Fig. 1). Side effects were observed in 30% of participants, but only one patient showed serious side effects and only three patients discontinued therapy due to adverse events. Both the efficacy and safety of evolocumab in the ECARA trial were comparable to data from previous randomized controlled clinical trials [2].
Fig. 1
HUYGENS: Evolocumab stabilizes atherosclerotic plaques
Up to 75% of ACS episodes are triggered by rupture of atherosclerotic plaques [4]. Among other things, the thickness of the fibrous cap (FCT), which encloses the soft lipid core, is decisive for the stability of the plaques. In vulnerable plaques, FCT is very low and is <65 μm [4]. Because a low LDL-C level favors stabilization of the fibrous cap, the HUYGENS study examined the extent to which the LDL-C-lowering effect of the PCSK9 mAb evolocumab affects atherosclerotic plaques [7–9]. In the double-blind, multicenter phase III study, 161 patients were randomized 1:1 to treatment with once-monthly evolocumab or placebo, each plus maximum tolerated statin dose, and plaques were assessed by optical coherence tomography [9]. The analysis shows that treatment with evolocumab plus optimized statin therapy for 48-52 weeks achieves almost twice the increase in minimal FCT compared with placebo plus statin therapy (42.7 μm vs. 21.5 μm, Fig. 2) [3]. At 12 months after ACS, only 12.5% of patients in the evolocumab arm still had a minimal FCT of <65 μm, compared with 30.2% in the placebo arm. In addition, lipid arc was reduced 83% more with evolocumab than with placebo (-57.5° vs. -31.4°). Here, the measurable change in plaques correlated with an LDL-C reduction of 80% in patients on evolocumab-statin therapy, compared with 39% on placebo-statin therapy [3]. The safety and tolerability of evolocumab in ACS patients were comparable to placebo. The most common treatment-related adverse events observed were injection site reactions and myalgias, but these did not differ significantly between the two groups [3].
Fig. 2 Increase in minimum FCT#
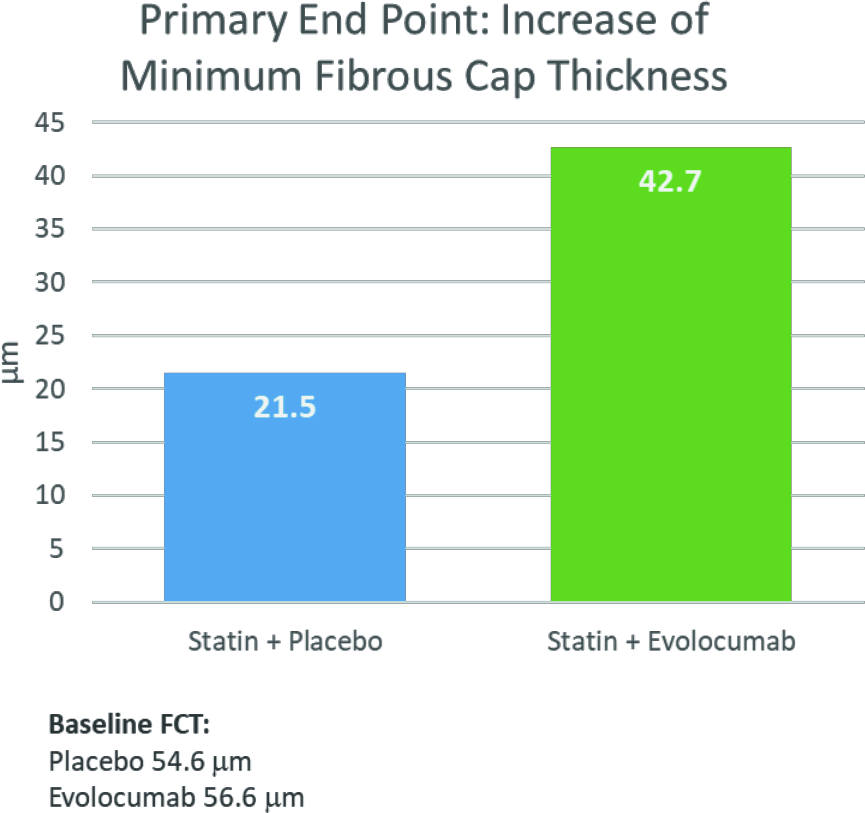
# after 48-52 weeks of treatment with evolocumab plus optimized statin therapy (42.7 μm) compared with placebo plus statin therapy (21.5 μm). FCT=thickness of fibrous cap.
Conclusion
The results of the ECARA and HUYGENS studies presented at the ESC Congress 2021 confirm that the addition of evolocumab to statin therapy is well tolerated and provides an additional benefit for the maximal reduction of LDL-C levels – and this also under real-world conditions in Swiss daily practice [2,3]. This is associated with increased stability of atherosclerotic plaques in ACS patients, which in turn may reduce cardiovascular risk [3].

This article was written with the financial support of Amgen Switzerland AG, Rotkreuz.
Literature:
1. McCormack T, Dent R, Blagden M: Very low LDL-C levels may safely provide additional clinical cardiovascular benefit: the evidence to date. Int J Clin Pract, 2016; 70(11): 886-897.
Nanchen D, et al: Effectiveness, adherence and safety of evolocumab in a Swiss multicenter prospective observational study. Poster presentation, abstract 81681, in European Society of Cardiology Congress. 2021: Barcelona, Spain and virtual.
3 Nicholls SJ, HUYGENS: Evolocumab and changes in plaque composition on OCT, in European Society of Cardiology Congress. 2021: Barcelona, Spain.
Virmani R, et al: Vulnerable plaque: the pathology of unstable coronary lesions. J Interv Cardiol 2002; 15(6): 439-446.
5. prevention of atherosclerosis 2020. Overview of the recommendations of the Lipids and Atherosclerosis Working Group (AGLA) of the Swiss Society of Cardiology (SGK) and the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). www.agla.ch/de/empfehlungen, last access: 16.03. 2021.
Mach F, et al: 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41(1): p. 111-188.
7. Kataoka Y, et al: Lipid Lowering Therapy to Modify Plaque Microstructures. J Atheroscler Thromb 2017; 24(4): 360-372.
8. Yano H, Horinaka S, Ishimitsu T: Effect of evolocumab therapy on coronary fibrous cap thickness assessed by optical coherence tomography in patients with acute coronary syndrome. J Cardiol 2020; 75(3): 289-295.
Nicholls SJ, et al: Assessing the impact of PCSK9 inhibition on coronary plaque phenotype with optical coherence tomography: rationale and design of the randomized, placebo-controlled HUYGENS study. Cardiovasc Diagn Ther 2021; 11(1): 120-129.
CH-REP-1121-00010