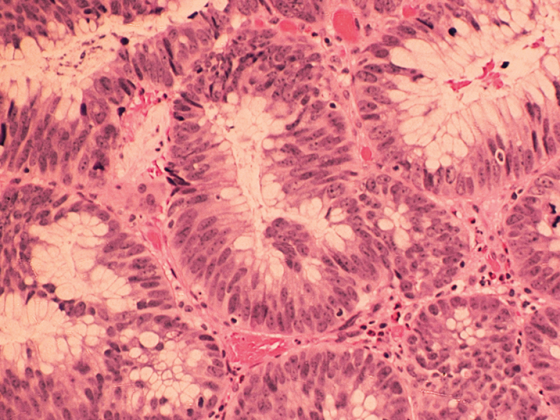
Various treatment approaches are available to effectively reduce the risk of osteoporotic fractures. In this context, a therapeutic option recommended by the Swiss Association Against Osteoporosis (SVGO/ASCO) for very high fracture risk [1] is characterized not only by its dual mechanism of action [2], but also by its exciting history of discovery.
In Switzerland, about 400,000 people, especially women, are affected by the insidious bone disease osteoporosis, which is characterized by the loss of bone substance and increased bone fragility [3]. Thus, one in three women aged 50 years and older suffers an osteoporosis-related fracture, often with drastic health, social, and financial consequences [3, 4]. In addition, the risk of a subsequent fracture doubles with the occurrence of a first fracture and is particularly high immediately thereafter [5].
Sclerostin antibody romosozumab recommended for very high risk of fracture
If there is a very high risk of fracture due to a recent fracture or other risk factors, such as low bone mineral density (BMD), the Swiss Association Against Osteoporosis (SVGO/ASCO) recommends, among other things, one year of treatment with romosozumab (EVENITY®) followed by antiresorptive therapy [1]. The monoclonal antibody has been approved since July 2020 for the treatment of severe osteoporosis in postmenopausal women at high risk of fracture [2, 6]. Its efficacy was demonstrated, among others, in the pivotal phase III ARCH trial, in which one year of treatment with romosozumab followed by the antiresorptive alendronate reduced fracture risk significantly more than alendronate monotherapy [7].
Want to learn more about the pivotal ARCH trial?
Click here
here
for a study summary or watch the video below!
Unique dual mechanism of action
The good efficacy of romosozumab is due to its unique dual mechanism of action: the monoclonal antibody primarily promotes bone formation, but at the same time counteracts bone resorption by binding to the glycoprotein sclerostin [2]. The latter inhibits bone formation by osteoblasts via inhibition of the canonical Wnt signaling pathway, on the one hand, and promotes bone resorption via stimulation of receptor activator of nuclear factor kappa-β-ligand (RANKL) production, on the other. When sclerostin is inhibited by romosozumab, bone formation by osteoblasts increases while bone resorption decreases [8]. Thus, trabecular and cortical bone mass are increased and both bone structure and bone strength improve. This reduces the overall risk of fracture [2].
Development of Romosozumab Based on Genetic Accidental Discovery
How did the discovery of the monoclonal antibody come about (Figure 1)? The development of Romosozumab began in 1958 with the first description of the rare hereditary skeletal dysplasia with osteosclerosis, which is characterized by excessive bone growth, particularly in the skull and mandible. The disease, which affects fewer than 100 people worldwide, has been called sclerosteosis since 1967 [9, 10]. The causes of its development were identified in 2001: Mutations in the SOST gene lead to a loss of sclerostin, which is associated with a disturbance of bone homeostasis. Consequently, the bones of people with sclerosteosis are thicker and stronger, making them potentially less susceptible to fracture [11].
From antibody screening to the clinic via space
This finding motivated the companies UCB and Amgen to develop a drug for diseases characterized by low bone mass. For example, in 2006, a screening of thousands of antibodies was performed in which romosozumab was identified as the best possible candidate for specifically binding and inhibiting sclerostin [2]. During the clinical trial phase, the efficacy of romosozumab was even tested in space in 2011 in collaboration with NASA. There, the lack of gravity can lead to a loss of bone mass in astronauts. In the course of the experiment, mice received either an injection of a version of the sclerostin antibody or placebo and were then sent into space for two weeks. Back on earth, the mice injected with sclerostin antibody exhibited greater bone formation and improved bone structure and strength than the placebo-injected mice [12].
Following these promising results, romosozumab demonstrated clinical efficacy first in Phase I and II studies and then in a global Phase III study program involving more than 11,000 patients. In 2020, romosozumab was finally approved as the first treatment option of its kind for severe osteoporosis in postmenopausal women [2, 6].

Figure 1: Developmental history of Romosozumab [2, 7, 9-11].
Conclusion
The development of romosozumab as an effective therapeutic option for the treatment of severe osteoporosis in postmenopausal women has a history spanning more than 60 years [2, 10]. Thanks to the discovery of the genetic causes of the rare hereditary disease sclerosteosis, the sclerostin antibody could be developed. This subsequently demonstrated its efficacy not only in a comprehensive clinical trial program but also in an experiment in space [7, 11, 12]. The use of romosozumab followed by bisphosphonates or denosumab has been recommended by SVGO/ASCO since 2020 for those at very high risk of fracture and has been shown to help prevent the occurrence of fractures [1, 7].
This text was produced with the financial support of UCB Pharma AG.
Brief technical information Evenity®
Literature
CH-P-RM-OP-2100032
Post online since 18.10.2021