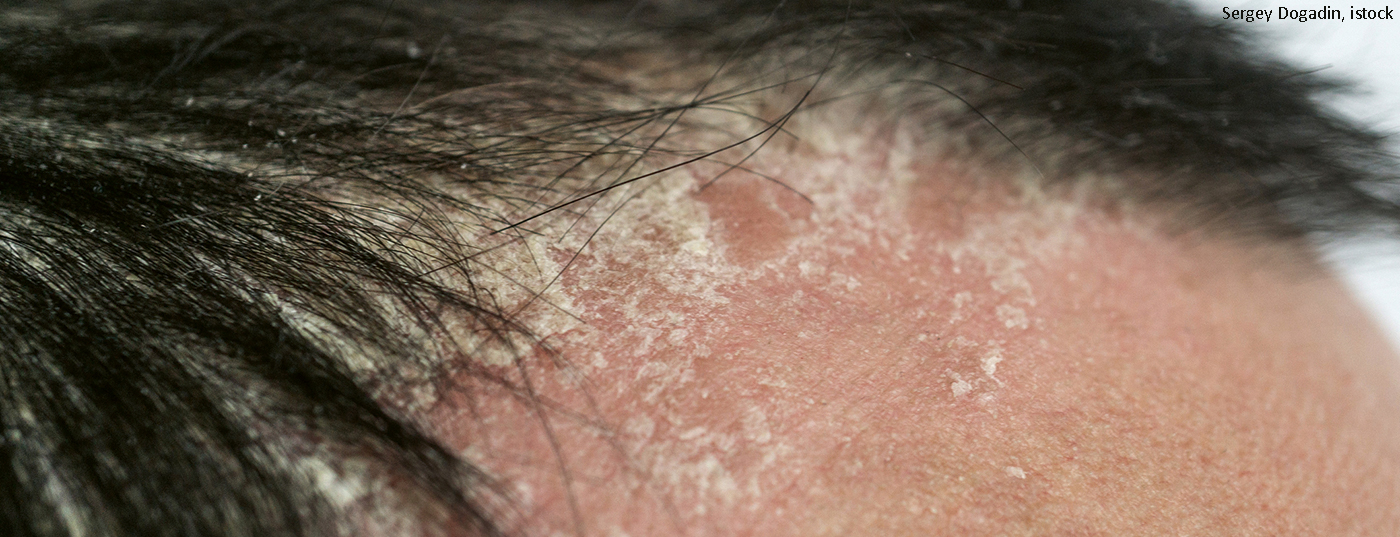
Visible pruritic lesions on the scalp are a major stress factor for many psoriasis patients. Topical preparations are used as first-line therapy, but these are not always effective, especially in cases of moderate to severe symptoms. In these cases, effective and tolerable systemic therapy is an important treatment alternative. Apremilast is one of the evidence-based treatment options.
Itching and stigmatization are often accompanied by a significantly reduced quality of life in patients with psoriasis capitis. While topical treatment may be sufficient for mild symptoms, this is often not the case for moderate to severe psoriatic lesions. Therefore, it is important that evidence-based systemic treatment alternatives are available for this patient population. These include the phosphodiesterase-4 (PDE-4) inhibitor apremilast (Otezla®). The small molecule antipsoriatic is available in oral form and has been shown to be effective in the treatment of patients with plaque psoriasis, including in subpopulations of patients with scalp involvement. This is shown by analyses of the phase III ESTEEM study 1 and 2 and the placebo-controlled LIBERATE study, which compared apremilast with etanercept [1–3]. The STYLE study is the first prospective randomized placebo-controlled study to evaluate the efficacy and safety of apremilast in patients with moderate to severe scalp psoriasis. The results were published last year in the Journal of the American Academy of Dermatology [4].
High proportion of patients achieved freedom from appearance
To evaluate the efficacy and safety of apremilast 30 mg (2×/d) for moderate to severe psoriasis capitis, a double-blind, placebo-controlled phase III study was conducted in adults with moderate to severe scalp psoriasis who had demonstrated an inadequate response to at least one topical psoriasis treatment. The primary endpoint was the proportion of patients who achieved a score of 0 (appearance-free) or 1 (near-appearance-free) on the Scalp Physician Global Assessment with a reduction of at least 2 points 16 weeks after baseline. Secondary endpoints were an improvement of at least 4 points in total itch (“Whole Body Itch”) and scalp pruritus (“Scalp Itch Numeric Rating Scales”, NRS), and improved quality of life (“Dermatology Life Quality Index”, DLQI). A total of 303 patients were randomized to the apremilast (n=201) or placebo (n=102) study arms. Under apremilast, significantly more patients met endpoints regarding Scalp Physician Global Assessment (43.3% vs. 13.7%), Scalp Itch NRS (47.1% vs. 21.1%), and Whole Body Itch NRS (45.5% vs. 22.5%).
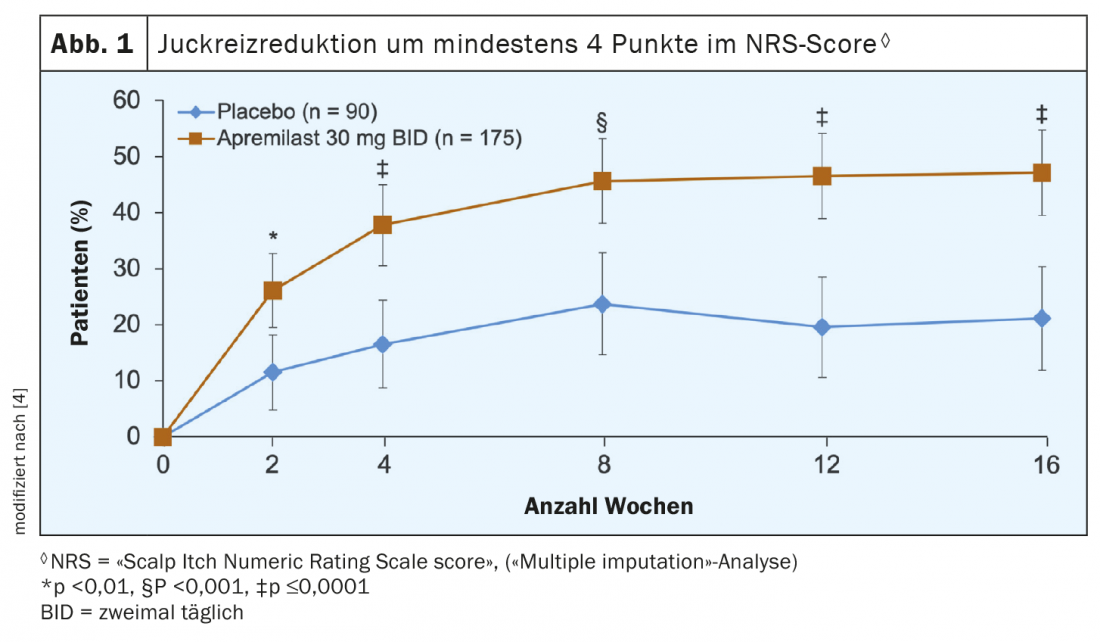
Itch reduction and improvement in quality of life as important outcomes.
To evaluate pruritus, patients rated itch symptoms in the scalp area using the NRS (“Scalp Itch Numeric Rating Scales”) measuring instrument on a scale from 0 (no itch) to 10 (itch of the greatest intensity imaginable). Figure 1 shows the data analysis, which indicates that the apremilast treatment resulted in demonstrably less itching in the scalp area. Thus, at all measurement time points up to and including week 16 after baseline, a significantly higher proportion of patients in the apremilast group achieved an improvement of at least 4 points in the NRS score. This is a significant target because chronic itching, similar to chronic pain, can significantly affect the quality of life and in certain cases leads to sleep deprivation, reduced performance and depressive disorders. The fact that patients also benefited from apremilast treatment in terms of quality of life is shown by a look at the scores in the “Dermatology Life Quality Index” (DLQI). The verum group showed a significantly greater improvement in least squares mean (LSM) scores compared with placebo (-6.7 vs. -3.8; p<0.0001). LSMs are means in which the influence of covariates on the dependent variable has been removed.
Overall, treatment was well tolerated; occasional side effects with apremilast included diarrhea, nausea, and headache.
Literature:
- Papp K, et al: Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM 1]). J Am Acad Dermatol. 2015; 73: 37-49.
- Paul C, et al: Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015; 173: 1387-1399.
- Reich K, et al: The efficacy and safety of apremilast, etanercept, and placebo, in patients with moderate to severe plaque psoriasis: 52-week results from a phase 3b, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017; 31: 507-517.
- Van Voorhees AS, et al: Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. JAAD 2020; 83(1): 96-103.
DERMATOLOGY PRACTICE 2021; 31(2): 34