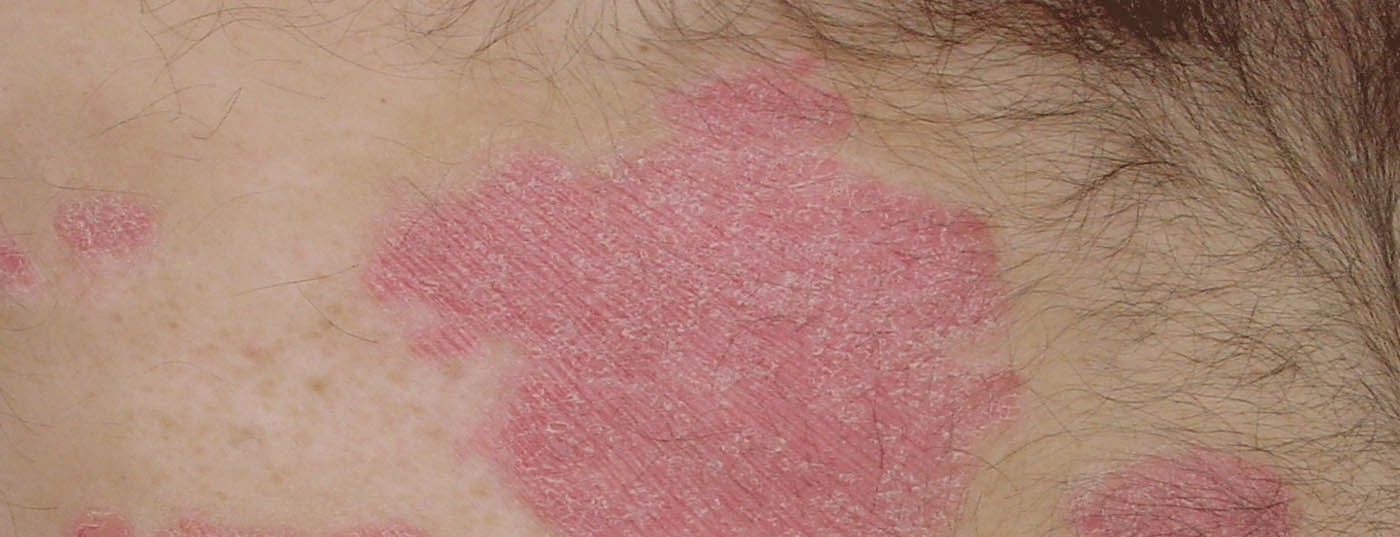
Selecting the appropriate treatment option among the multitude of available antipsoriatic agents is one of the greatest challenges today. Empirical studies show that the therapeutic response of different biologics varies depending on patient characteristics. Patient-oriented selection criteria can counteract the problem of secondary loss of efficacy. This is especially true for first biologic therapy, as this has been shown to be an important influencing factor for “drug survival.”
According to current understanding, psoriasis is regarded as an inflammatory systemic disease mediated by T-lymphocytes, in which autoimmunity against endogenous antigens of the skin occurs due to a genetically determined dysregulation of the immune system. Comorbid extracutaneous manifestations are also common. Through activation of the immune system, T helper (Th) cells in the skin produce various cytokines that act on epidermal keratinocytes, leading to the characteristic excessive proliferation and abnormal differentiation of keratinocytes, as well as infiltration with inflammatory cells [1]. As is now known, Th17 cells, which produce various inflammation-mediating messengers, play a key role in the pathomechanism of psoriatic disease [2]. Both IL17 and IL23 inhibitors, which are both part of the newest generation of biologics, interact with the Th17 immune response. However, there are some notable differences between these two groups of monoclonal antibodies. This is shown by the findings of several empirical studies presented at the 2020 EADV Annual Congress.
Identify predictors of treatment response
Currently, one of the greatest challenges in the systemic treatment of psoriasis is the management of secondary loss of efficacy of biologics, i.e. a decrease in the therapeutic effect after a certain treatment period [3]. In particular, the choice of the first biologic should be tailored as well as possible to individual patient characteristics, since “drug survival”, i.e. the duration from the initiation of therapy until its discontinuation or switch to another active substance, is influenced by this [4]. Predictors of “drug survival”, may be useful for choosing between different biologics [5,6]. Therefore, stratification is important to derive criteria for which patients are likely to show better response to treatment with anti-IL17 and which are better suited for anti-IL23 therapy.
A treatment option that is as well suited as possible to the individual patient can also be an important factor in terms of therapy motivation. If a patient has already had several unsuccessful therapy attempts with different biologics, the fear of a renewed loss of efficacy can have a negative influence on adherence. Systemic treatment options whose efficacy lasts as long as possible would be desirable. Among other things, this is the focus of long-term studies on the durability of treatment effects. Some relevant empirical findings are already available at this point in time.
Anti-IL17 vs. anti-IL23 therapy: patient-oriented selection criteria.
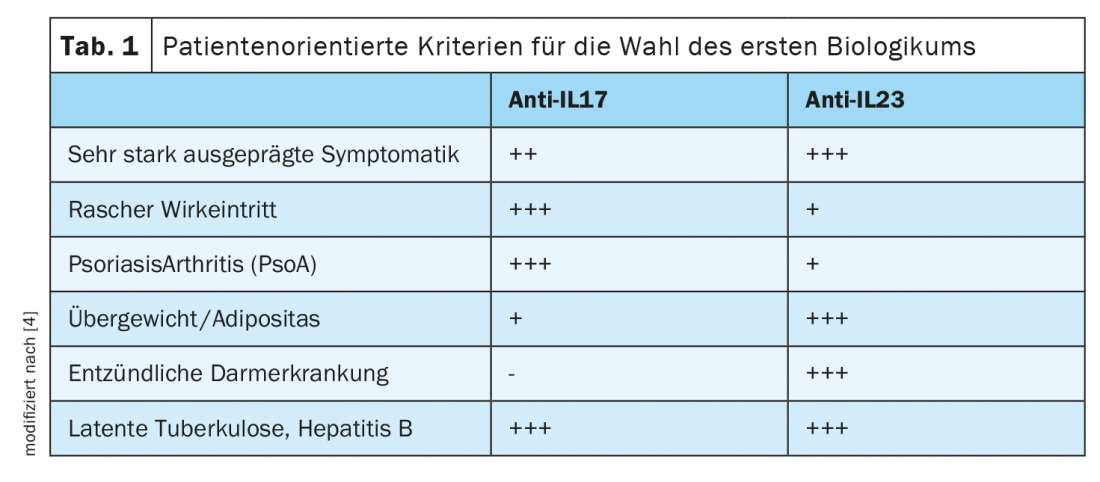
IL17 and IL23 inhibitors are among the newest generation of biologics. Currently approved in Switzerland are the IL17A inhibitors secukinumab (Cosentyx®) and ixekizumab (Taltz®) and for the IL23p19 antagonists risankizumab (Skyrizi®), guselkumab (Tremfya®), and tildrakizumab (Ilumetri®). Ustekinumab (Stelara®) is also available as an IL12/IL23 inhibitor [7]. Differentiating criteria for anti-IL17 vs. anti-IL23 treatment include the severity of psoriatic disease, the speed of onset of action of the biologic, and whether psoriatic arthritis, obesity, or comorbid inflammatory bowel disease are present, as explained by Prof. Curdin Conrad, MD, Department Head of the Polyclinic and Psoriasis Center of the Department of Dermatology, Lausanne University Hospital, at the 2020 EADV Annual Congress [4]. (Tab.1).
Regarding speed of onset of action, the head-to-head study IXORA-R, published in 2020, demonstrated that a faster PASI response was achieved under the IL17 inhibitor ixekizumab than under the IL23p19 inhibitor guselkumab. This was true for PASI50 and PASI75 as well as for PASI90 and PASI100 [8]. One conclusion was that the IL17 inhibitor was superior to the IL23 inhibitor in terms of efficacy. In contrast, the head-to-head study ECLIPSE, which also compared anti-IL23p19 vs. anti-IL17, concluded that IL23 inhibition was superior to IL17 blockade [9]. Regarding patient characteristics, the average BMI of study participants was 30, with over 40% of patients meeting criteria for obesity (BMI ≥30) [10,11]. Several years ago, it was found that IL17 and IL23 blockade underlie different mechanisms of action with respect to TH17 cells by demonstrating, among other things, different effects on symptoms of inflammatory bowel disease [12]. Therefore, psoriatics with comorbid inflammatory bowel conditions should be more likely to consider an IL23 inhibitor. In contrast, if psoriatic arthritis is present, IL17 inhibitors are a very interesting therapeutic option, as recent evidence of efficacy of secukinumab and ixekizumab in this patient population shows [13,14].
Source: EADV Annual Meeting 2020
Literature:
- Sator P: State of the Art: Therapy of Psoriasis vulgaris. Focus: Skin and Rheumatism, FdR 01|2019, Apr. 16, 2019.
- Eyerich K: Psoriasis – Where are we heading? Key Lecture 3, slide presentation, Prof. Dr. med. Kilian Eyerich, TU Munich, SGDV Annual Meeting, Basel 20.09.2019.
- Yawalkar N: Psoriasis without Symptoms – the New Hope for Patients. SGDV Annual Meeting, Basel 20.09.2019
- Conrad C: Targeting IL17 & IL23. Presentation, EADV (Virtual) 29.10.20.
- Egeberg A, Bryld LE, Skov L: Drug survival of secukinumab and ixekizumab for moderate-to-severe plaque psoriasis. J Am Acad Dermatol 2019; 81(1): 173-178.
- Mourad A, et al: Factors predicting persistence of biologic drugs in psoriasis: a systematic review and meta-analysis. Br J Dermatol 2019; 181: 450-458.
- Swiss Drug Compendium, www.compendium.ch (last accessed Feb. 17, 2021).
- Blauvelt A, et al: A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. British Journal of Dermatology 2020, https://onlinelibrary.wiley.com/doi/10.1111/bjd.19509
- Reich K et al: Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019; 394(10201): 831-839.
- Armstrong A: Consistency of Response by age in Patients With Moderate-to-Severe Psoriasis Treated With Guselkumab vs. Secukinumab: Week 48 Results From the ECLIPSE Study Trial. P1594, EADV 2019, https://eadvprogram.m-anage.com/
- Armstrong AW et al. guselkumab demonstrates greater efficacy compared to secukinumab across body weight quartiles and body mass index categories: Week 48 results from the ECLIPSE trial. Abstract P1631. EADV Congress, 9-13 October, 2019.
- Patel DD, Kuchroo VK: Th17 Cell Pathway in Human Immunity: Lessons from Genetics and Therapeutic Interventions. Immunity 2015; 43(6): 1040-1051. doi: 10.1016/j.immuni.2015.12.003.
- McInnes IB, et al: Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet 2020, DOI: 10.1016/S0140-6736(20)30564-X.
- Smolen J, et al: A Head-to-Head Comparison of Ixekizumab and Adalimumab in Biologic-Naïve Patients with Active Psoriatic Arthritis: Efficacy and Safety Outcomes from a Randomized, Open-Label, Blinded Assessor Study Through 52 Weeks. Abstract Number: L20, 2019 ACR/ARP Annual Meeting.
DERMATOLOGIE PRAXIS 2021; 31(1): 36-37 (published 2/21, ahead of print).