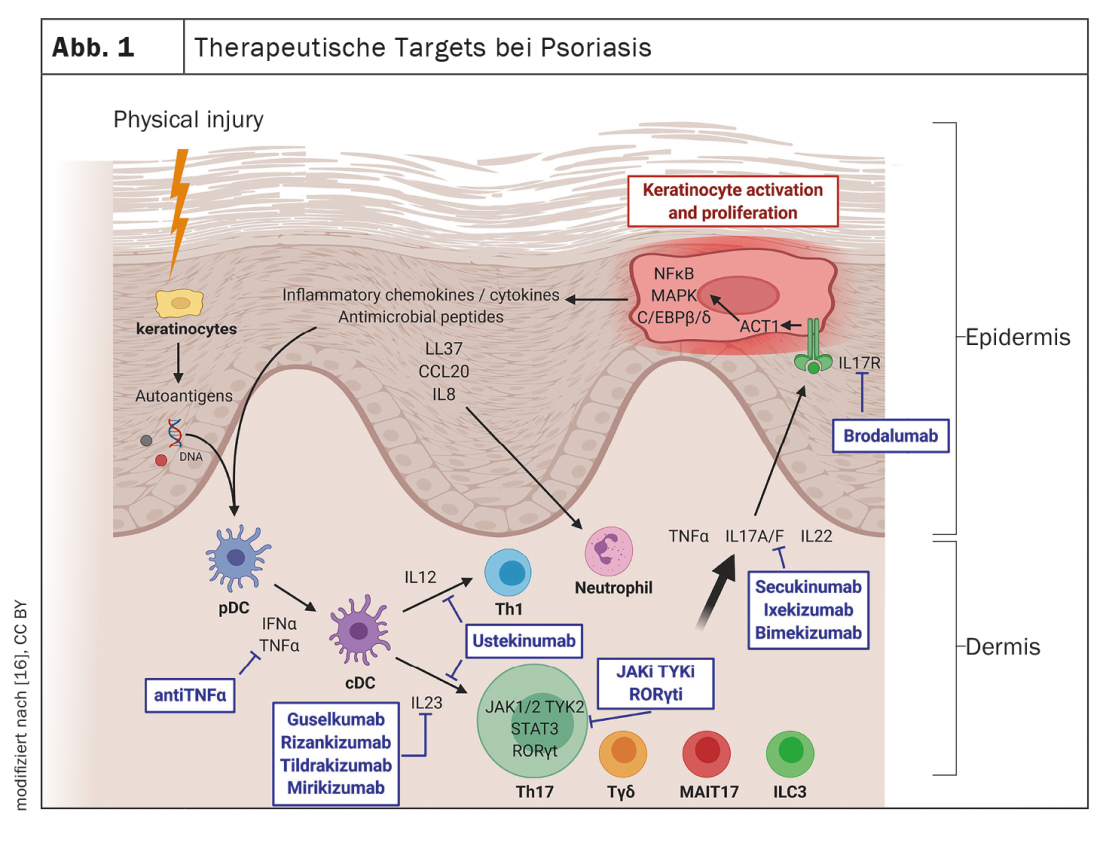
Today, psoriasis is considered an inflammatory systemic disease in which proinflammatory cytokines play an important role. Several inflammatory circuits are involved in the pathogenesis of psoriasis. Cytokines of the IL-23 and IL-17 families are established targets, but other therapeutic targets are also being explored.
The efficacy and safety of systemic immunotherapy has improved significantly in recent years with the introduction of new agents from the IL-17 and IL-23 inhibitor classes. “We have witnessed a rapid increase in the number of preparations in recent years,” says Sylvie Haase, MD, a specialist in skin and venereal diseases in a dermatological group practice in Constance, Germany [2]. A wide range of systemic agents are now available to address different targets, and as molecular pathomechanisms continue to be deciphered, this trend is expected to continue in the future (Fig. 1). Increasingly, the challenge is to match patients to appropriate treatment options. It turns out that among patients with moderate to severe psoriasis, there are those who respond very well to the first systemic therapy option and stay on the same treatment for years, but also some in whom psoriasis proves refractory, the speaker explained.
Analyses of response to biologics: what can be inferred?
The core pathological feature of psoriasis is chronic skin inflammation leading to uncontrolled proliferation and dysfunctional differentiation of keratinocytes. At the molecular level, the central pathophysiological role of the TNF-α-IL-23-Th17 axis is well characterized. The monoclonal antibodies currently approved in Switzerland for the treatment of psoriasis are directed against the following cytokines: TNF-α, interleukin(IL)-12/IL-23, IL-23, IL-17A, or IL-17A/F [1]. According to network meta-analyses, the IL-17 inhibitor (IL-17-i) and IL-23 inhibitor (IL-23-i) groups show higher efficacy in terms of PASI*-90 and PASI-100 compared to TNF-α-i [8]. Regarding IL-23-i, Dr. Haase explains, “The onset of action is somewhat slower compared with IL-17 inhibitors, but the compounds do not have a specific side effect spectrum.” In Switzerland, the IL-17A-i secukinumab and ixekizumab are currently approved, as well as more recently the IL17A/F-i bimekizumab, and among the IL-23-i, guselkumab, risankizumab and tildrakizumab are on the market.
* PASI = Psoriasis Area and Severity Index
In a cohort study, a research team from Denmark analyzed the response to different biologics [3]. Results published in 2022 in the Journal of the European Academy of Dermatology and Venereology showed that of the total 3280 patients included (mean age 45.0 years, 37% female), 6.3% were classified as super-responders and 6.5% as refractory to treatment. The latter had a higher mean BMI compared to the other patients (32.2 vs. 29.4, p<0.0001), while the super-responders had fewer comorbidities and a higher socioeconomic status.
IL-17A/F inhibitor bimekizumab is a new player
That IL-17A plays a central role in the pathogenesis of psoriasis has been repeatedly demonstrated by the successful use of the corresponding inhibitory antibodies. However, IL-17F is also overexpressed in skin and synovial tissue of psoriasis sufferers [4]. Bimekizumab, an IgG1 monoclonal antibody that selectively inhibits interleukin IL-17F and IL-17A, has recently become available in Switzerland. In the three pivotal studies, bimekizumab showed good efficacy and performed better than the IL-12/IL-23 antagonist ustekinumab and the TNF-α inhibitor adalimumab with respect to the co-primary endpoints PASI-90 and IGA# 0/1 at week 16 [5–7]. The safety profile of bimekizumab is characterized by known class effects of IL-17 inhibition; common side effects include oral candidiasis, for example. A novel trivalent nanobody against IL-17A and IL-17F currently being explored for use in psoriasis is sonelokimab. Promising results have been obtained in phase I and II studies [4]. Nanobodies are recombinant, antigen-specific antibodies that consist only of the variable domain necessary for antigen binding and are thus comparatively small.
# IGA=Investigator’s Global Assessment: patients with IGA: 0 = appearance-free skin, 1 = mild skin symptoms.
Several orally or topically applied agents are also in the pipeline
The investigation of new antipsoriatic agents is a rapidly developing field of research. The following systemic agents have been newly approved for plaque psoriasis by the US Food and Drug Administration (FDA) this year (Table 1):
- Deucravacitinib (BMS-986165) is a highly selective TYK2 inhibitor in oral dosage form for once-daily use in moderate-to-severe plaque psoriasis (FDA approval 09/09/22) [9,10].
- Roflumilast (ARQ-151) is a topical PDE-4 inhibitor for the treatment of plaque psoriasis in patients ≥12 years of age (FDA approval 07/29/22) [11–13].
- Tapinarof-Crème 1% (Vtama) is an aryl hydrocarbon receptor modulator for the treatment of plaque psoriasis in adults (FDA approval 05/24/22) [18,19].
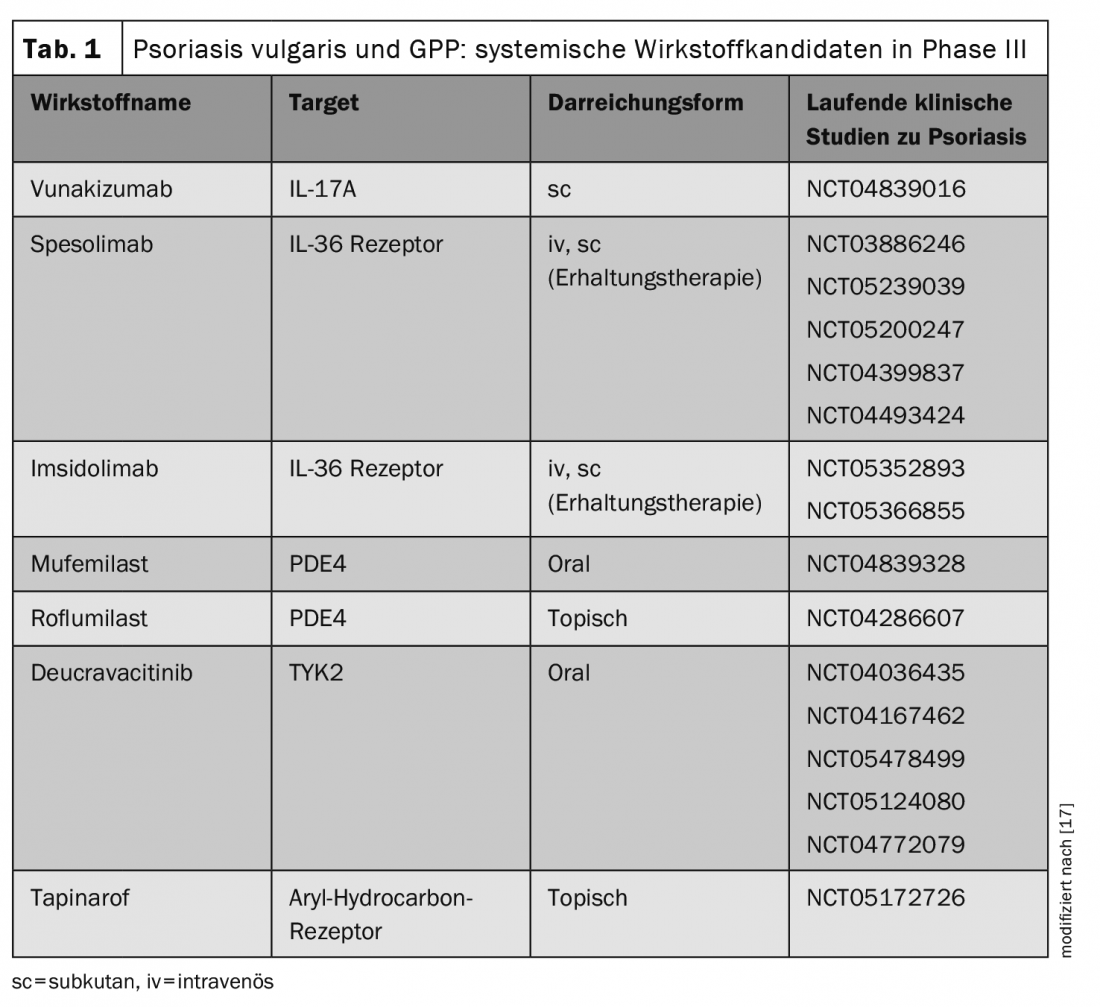
In addition, spesolimab, a humanized monoclonal antibody against IL36-R was approved by the FDA for the treatment of GPP (Generalized Pustular Psoriasis) flares (FDA approval 01/09/22). The approval decision is based on the Phase II Effisayil-1 trial [14,15]. Other innovative systemic agents are currently in advanced phases of clinical development (Table 1) [17]. It is therefore possible that the treatment spectrum could also be expanded in Europe in the not too distant future to include further system therapeutics. Especially for refractory patients, new treatment alternatives are welcome. Regarding the side effect profiles, Dr. Haase points out that the known substance class effects of JAK inhibitors include a slightly increased number of upper respiratory tract infections and herpes simplex, and acne-like exanthema may occasionally occur. Long-term data are needed to better understand the differences between treatment options and to make patient-centered choices.
Congress: Bodensee Dermaconsil
Literature:
- Lauffer F, et al: IL-17 family cytokines in psoriasis. JDDG; 1610-0379/2020/1807
- “Psoriasis – is there anything new?”, Sylvie Haase, MD, 3rd Bodensee Dermaconsil, Oct. 22, 2022.
- Loft N, et al: Prevalence and characterization of treatment-refractory psoriasis and super-responders to biologic treatment: a nationwide study. J Eur Acad Dermatol Venereol 2022; 36(8): 1284-1291.
- Iznardo H, Puig L: Dual inhibition of IL-17A and IL-17F in psoriatic disease. Ther Adv Chronic Dis 2021 Aug 12;12:20406223211037846
- Warren RB, et al: Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med 2021; 385: 130-141.
- Reich K, et al: Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet 2021; 397: 487-498.
- Gordon KB, et al: Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet 2021; 397: 475-486.
- Yasmeen N, et al: Targeted therapies for patients with moderate-to-severe psoriasis: a systematic review and network meta-analysis of PASI response at 1 year. J Dermatolog Treat 2022; 33(1): 204-218.
- Boerner LK. Hybrid meeting divulges structures of drug candidates [Internet]. Chemical & Engineering News. 2022. Available from: https://cen.acs.org/acs-news/acs-meeting-news/Hybrid-meeting-divulges-st…,(last accessed 11/15/2022).
- Chimalakonda A, et al: Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with Janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb) 2021; 11(5): 1763-1776.
- Lebwohl MG, et al: Trial of roflumilast cream for chronic plaque psoriasis. N Eng J Med 2020; 383(3): 229-239.
- Arcutis Biotherapeutics. FDA approves Arcutis’ ZORYVE (roflumilast) Cream 0.3% For the treatment of plaque psoriasis in individuals age 12 and older [Internet]. 2022. Available from: www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-cream-0-3-for-th…,(last accessed 11/15/2022).
- Lebwohl M, et al: Safety and efficacy of once-daily roflumilast Cream 0.3%, a potent phosphodiesterase-4 inhibitor for the treatment of psoriasis in the DERMIS-1 and DERMIS-2 phase 3 trials. Poster presented at:30th EADV Congress, Sept 29-Oct 2, 2021; virtual
- Boehringer Ingelheim. FDA approves the first treatment option for generalized pustular psoriasis flares in adults [Internet]. 2022. Available from: www.boehringer-ingelheim.us/press-release/fda-approves-first-treatment-o…,(last accessed 11/15/2022).
- Bachelez H, et al: Trial of spesolimab for generalized pustular psoriasis. N Engl J Med 2021; 385(26): 2431-2440.
- Bugaut H, Aractingi S: Major Role of the IL17/23 Axis in Psoriasis Supports the Development of New Targeted Therapies. Front Immunol 2021;12:621956. www.frontiersin.org,(last accessed Nov 15, 2022).
- Drakos A, Vender RA: Review of the Clinical Trial Landscape in Psoriasis: An Update for Clinicians. Dermatol Ther (Heidelb) 2022, https://doi.org/10.1007/s13555-022-00840-9,(last accessed Nov 15, 2022).
- Keam SJ: Tapinarof cream 1%: first approval. Drugs 2022; 82(11): 1221-1228.
- Lebwohl MG, et al: Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med 2021; 385(24): 2219-2229.











