The increasing age of the population and the gratifying progress in oncological therapy mean that rheumatologists are increasingly confronted with patients who have a previous malignant disease but still require a guideline-based basic therapy. Basic therapeutics are not infrequently immunosuppressive and one of the main roles of the immune system is to recognize and eliminate malignant cells. Accordingly, physicians and patients are concerned that basic therapy increases the risk of tumor recurrence.
The increasing age of the population and the gratifying progress in oncological therapy mean that rheumatologists are increasingly confronted with patients who have a previous malignant disease but still require a guideline-based basic therapy. Basic therapeutics are not infrequently immunosuppressive and one of the main roles of the immune system is to recognize and eliminate malignant cells. Accordingly, physicians and patients are concerned that basic therapy increases the risk of tumor recurrence. Especially in the case of tumor necrosis factor inhibitors, this concern is further fueled by unfortunate naming.
Increasing abundance of data, difficult interpretation
In order to make such therapeutic decisions, it is usually of little help to deal with the impressive and exciting wealth of data on in vitro experiments or animal models on the interplay between tumor cells and the immune system. As mentioned at the beginning, tumor surveillance is an important function of the immune system; inflammatory processes lead to the destruction of degenerated cells. On the other hand, inflammation can also contribute to tumor proliferation and inhibit tumor surveillance. Chronic inflammation is known to increase the risk of lymphoma [1]. Similar observations exist for certain solid tumors depending on tumor stage [2–4]. Depending on the microenvironment, the same cell groups or cytokines can exert very different effects, i.e. both tumor-inhibiting and tumor-promoting. For example, TNF-alpha, otherwise known primarily as a tumor inhibitory cytokine, can stimulate regulatory T cells in ovarian cancer, which are associated with a higher risk of progression because they likely suppress tumor surveillance [5].
The studies that otherwise provide the best evidence in medicine, namely randomized controlled trials, are only suitable to a very limited extent, even after meta-analysis, to clarify the question of whether the respective preparation increases the risk of tumor recurrence. There are several reasons for this:
- The case number calculation and design of pivotal trials are primarily guided by evidence of effectiveness. As a result, case numbers are too small and follow-up is too short to capture certain safety aspects.
- Malignant diseases are rare events that usually occur only after a long latency period. Under cyclophosphamide, these latencies range from 10 to 15 years.
- Previous malignant disease (with the exception of non-melanocytic skin cancers) and often advanced age are exclusion criteria. Also, older patients not infrequently have comorbidities or logistic limitations, so patients at high risk for cancer are usually underrepresented in studies.
Relatively few studies exist that focus primarily on the risk of tumor recurrence during immunosuppressive therapy. These are evaluations of national registers or insurance databases. However, since the participating physicians themselves determine in these studies which therapy a patient with previous malignant disease receives, distortions can occur in that preparations which the physician considers to have a high tumor-promoting potential are used more cautiously in patients with a high risk of tumor recurrence. This bias is attempted to be addressed in the statistical analysis of these studies by adjusting for the usual risk factors for tumor recurrence (e.g., initial tumor stage). The advantage of insurance databases is the high number of cases; the disadvantage is the lack of detailed information on the individual patient, which makes adjustment considerably more difficult.
In the absence of direct evidence on tumor recurrence risk, most preparations attempt to be guided by the risk of new incident malignancies in randomized trials, registries, and insurance databases, and extrapolate this risk to recurrence risk.
Given these limitations in the data, there are also other opportunities for gaining knowledge:
- One looks at studies of immunosuppressive therapy in at-risk populations that have a higher likelihood of malignancy: for example, patients with precancerous conditions such as cervical intraepithelial neoplasia or monoclonal gammopathies.
- For studies in which different dosages are used, a dose effect may be examined.
- Non-melanocytic skin cancers (NMSCs) are malignancies that are more common and for which differences have been seen even in studies with shorter follow-up [6]. These entities could serve in a limited way as surrogates for overall cancer risk, especially since they are usually not exclusion criteria for randomized-controlled trials. However, NMSCs are often not fully captured even in studies, which is the reason why they are often excluded from malignancy risk considerations.
When considering studies on the incidence of malignant disease, particular attention is paid to the respective control group. Comparisons with the normal population are less helpful because rheumatoid inflammatory diseases often have increased incidences of certain malignancies independent of influences of therapy, such as bronchial carcinoma in rheumatoid arthritis [7], lymphoma in Sjögren’s syndrome [8], and cervical carcinoma in systemic lupus erythematosus [9].
Statements in guidelines and recommendations
There are many national and international guidelines for the treatment of rheumatoid arthritis that address the issue of malignancy history in varying detail [10]. The clearest recommendations on this were made by the American College of Rheumatology in 2012 and 2015 [11,12]. In the older recommendations from 2012, a distinction was still made – as in most other guidelines – as to whether the interval between the end of curative tumor therapy and the start of basic therapy was more or less than 5 years. At that time, it was only possible to refer to the usual time of highest tumor recurrence probability and to data from the organ transplantation field [13]. Beyond 5 years, any biologic therapy (mainly TNF inhibitors at that time) was considered safe. Within the 5 years, rituximab was given preference without any justification by studies at the time, with the exception of CD20+ B-cell neoplasms. Even with all the methodological problems, the only study published to date from the hemato-oncology field tended to indicate that patients who received rituximab in addition to autologous stem cell transplantation had a higher risk of secondary malignancies than patients without rituximab therapy [14]. Only subsequently have registry analyses been published that actually suggest rituximab is relatively unproblematic as detailed below.
However, in the most recent version of the 2015 ACR recommendations, the distinction depending on the interval between tumor and rheumatoid therapy was abandoned. Thus, in patients with a solid malignancy, the basic therapy can be treated without restriction as in RA patients without a history of malignancy. Otherwise, prioritizations for DMARDs were made for skin cancer and lymphoproliferative disorders that were so lacking in evidence or contrary to the non-cited evidence already available at the time that they will not be reproduced here. However, the fact that these two malignant disease groups require special attention will be illustrated in the following.
Direct evidence on tumor recurrence risk during rheumatologic therapy.
In national registries of RA patients, patients with a history of malignancy were compared in terms of case-control studies with patients who had similar baseline therapies and no history of malignancy. In most cases, an attempt was made to adjust for risk factors for tumor recurrence such as disease activity or obesity. The first meaningful data came from the German RABBIT registry [15] and the UK Biologics Registry [16]. In addition, analyses from the US CORRONA registry [17], the Danish DANBIO registry [18], and – recently published – the Swedish ARTIS registry [19] should be mentioned. Recently, a meta-analysis of 11 studies was published by Xie and colleagues that included above-mentioned registries [20]. The data are only usable for TNF-inhibitors and rituximab. Encouragingly, in each case, there was no increased risk of relapse for TNF inhibitors or rituximab compared with patients receiving conventional synthetic basic therapeutics (csDMARDs). It should be noted that in most studies the interval between oncologic and rheumatologic therapy was on average significantly longer than 5 years, with two exceptions: Rituximab was used earlier in the German registry, following the older ACR recommendation, and the Danish registry allowed comparison of patients with an interval to TNF inhibitor therapy of more or less than 5 years. Here, numerically even a slightly lower risk for tumor recurrence at shorter interval was shown even after adjustment for initial tumor stages. However, it was shown here that actually the physicians participating in the registry used biologics rather in initially lower tumor stages. A meta-analysis of 16 studies in different autoimmune diseases (RA, inflammatory bowel disease, and psoriasis) also failed to demonstrate a statistically significant difference in tumor recurrence incidence at intervals greater or less than 6 years. The analysis mainly involved TNF inhibitors, thiopurines, and methotrexate [21]. However, because most registry data show long intervals between oncologic and rheumatologic therapy on average, and registry studies may be subject to substantial bias, the length of the interval should still be factored into rheumatologic therapy decisions.
Probably the most informative study on tumor recurrence risk with a short interval is the analysis of US insurance data with a very large number of cases by Mamtani and colleagues [22]. These were patients with rheumatoid arthritis (>90%) or inflammatory bowel disease who received methotrexate, thiopurines, TNF inhibitors, combinations thereof, or no baseline therapy after curative surgery for breast carcinoma. In more than 90% of patients, TNF inhibitors were restarted within one year after surgery, and even with continuous administration of TNF inhibitors, no increased risk of breast carcinoma recurrence could be observed. However, follow-up was only 3.4 years. Because breast carcinoma recurrences can occur much later than 5 years after surgery, special caution and a wait-and-see approach to initiating basic therapy have been advised to date. This study can be used to some extent as a counterargument to this assessment. In the UK registry, relatively large numbers of cases also showed no increased incidence of new breast carcinomas with TNFIs compared with csDMARDs [23].
Data on individual substances
A detailed description of the data on all substances commonly used in rheumatology would go too far in the context of this review article. Therefore, in the following, the data situation will be summarized according to Table 1 and the tumor risk of the individual substances or substance groups will be evaluated. Only current literature is cited. For a more in-depth reading, we refer here to significant reviews [24–27].

It should be mentioned at the outset that only for cyclophosphamide is there a clear signal of an increased tumor risk or tumor recurrence risk – and that only at higher cumulative doses. The immunomodulatory or immunosuppressive therapies otherwise commonly used in rheumatology are likely to have at most a minor – and in some situations even a favorable – influence on these risks. With increasing evidence, the positive picture tends to be confirmed.
In Table 2, against the background of the relevant literature, an attempt was made to assess the extent to which single substances of rheumatologic therapy might affect the risk for the occurrence of new malignancies or malignancy recurrences. While this is a considerable simplification of the complex subject matter, it should serve as a quick orientation and a starting point for further discussion and research. The overall judgment about a substance should always be based on both the quality of the data and the resulting evidence of malignancy risk. Thus, when in doubt, one would rather choose the one with the better data in the case of alternative substances with probably low risk.
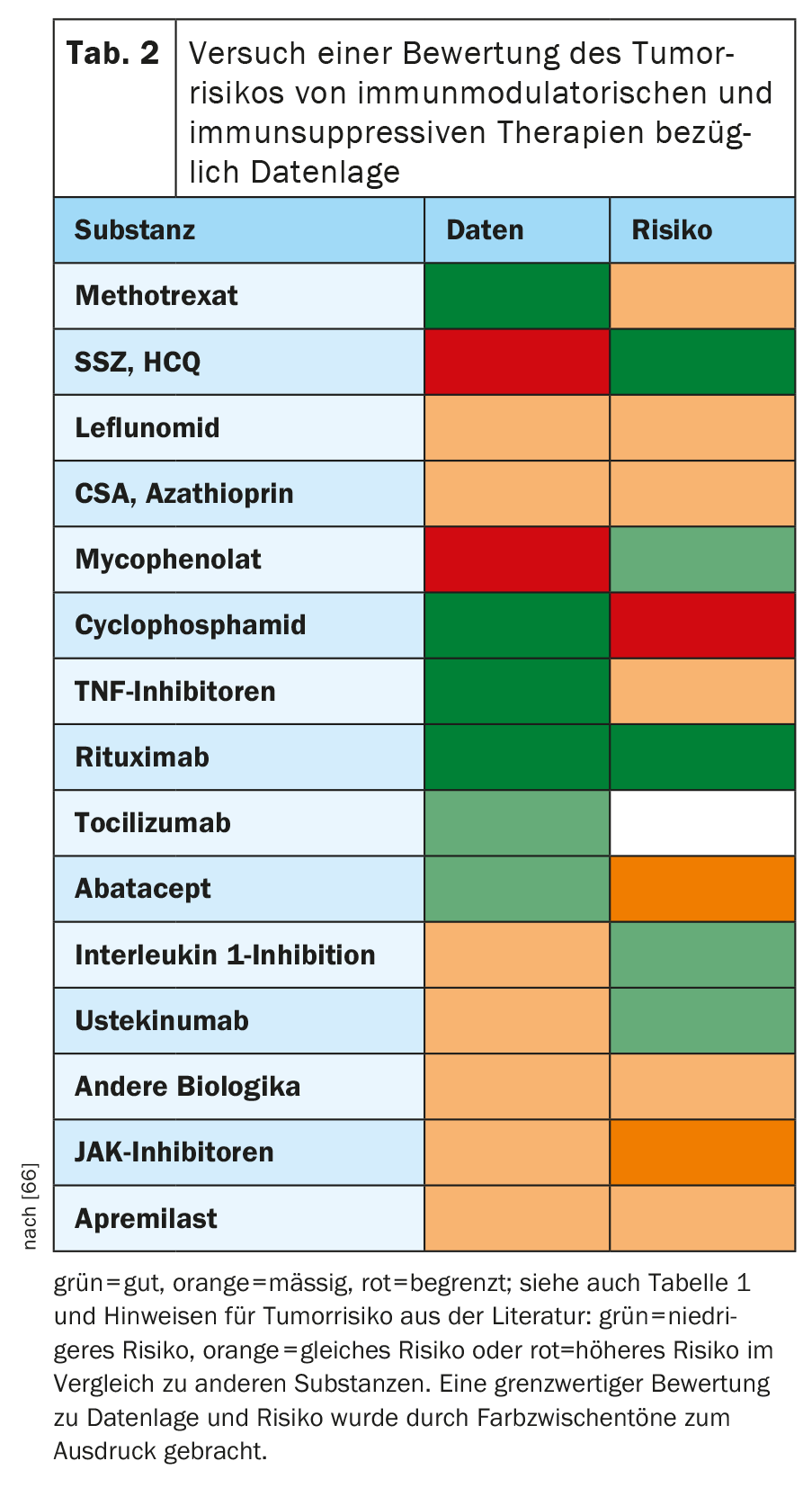
Methotrexate: Data situation: good.
Methotrexate is used as maintenance therapy for acute lymphoblastic leukemias. A cumulative dose-dependent slightly increased risk for non-melanocytic skin cancer must be assumed, which was again shown in the large randomized study on the use of methotrexate in cardiovascular risk patients [28,29]. The accumulation of lymphoproliferative disorders described mainly by Japanese authors, which sometimes remit spontaneously after discontinuation of methotrexate [30], is not confirmed in the synopsis of other detailed registry data [31].
Leflunomide, hydroxychloroquine, sulfasalazine: Data: moderate for leflunomide, poor for hydroxychloroquine and sulfasalazine.
Leflunomide has tumor inhibitory effects, at least in vitro. The numerical clustering of pancreatic cancer in the RABBIT registry with leflunomide was not found in other registries [32].
For hydroxychloroquine, a study in SLE and with low numbers of malignant events suggests a protective effect against malignancies [33]. A recent study on collagenoses suggests only a beneficial effect on the incidence of metastatic tumor disease [34]. Oncologic therapies in combination with hydroxychloroquine are discussed because of the effect on autophagocytosis.
Sulfasalazine, similar to mesalazine, may reduce the risk of colon cancer in ulcerative colitis [35].
Apremilast: Data situation: moderate.
FDA adverse event reports suggest that apremilast has an even lower tumor risk than ustekinumab [36]. Similar to hydroxychloroquine and sulfasalazine, an unfavorable effect on tumorigenesis seems unlikely based on the mechanism of action alone.
Azathioprine, ciclosporin A, mycophenolate: Data: moderate.
Evidence exists that combined therapy of azathioprine with infliximab may result in increased incidence of the very rare hepatosplenic T-cell lymphomas in men with inflammatory bowel disease [37]. The clustering of myeloid neoplasms by azathioprine in different autoimmune diseases postulated in a recently published study was not found in other studies and is probably due to the fact that very heterogeneous populations were compared [38]. For both azathioprine and ciclosporin A, the risk for NMSC appears to be increased [39], for CSA especially in association with light therapy for psoriasis [40]. The data on azathioprine from different registries on the incidence of overall cancer, urothelial carcinoma, and lymphoma is conflicting, although no strong signal emerges. For MMF, studies exist mainly from the organ transplantation field. Here, the risk of cancer appears to be somewhat lower overall for skin tumors and for post-transplant lymphoproliferative disorders compared with other post-transplant immunosuppressive regimens [41].
Cyclophosphamide: Data situation: good.
Depending on the cumulative dose of cyclophosphamide, the risk of new malignancy or tumor recurrence is increased. Most studies give values between 20 and 30 g as the limit above which this risk is calculated as significantly increased [42]. In fact, a study of SLE patients in South Korea already found an increased risk of incidences of malignancy at a cumulative dose of >6 g [43]. Even this value, however, is not achieved by modern cyclophosphamide protocols such as the Euro-Lupus protocol.
Biologics
TNF inhibitors: Data situation: very good.
A slightly increased risk for NMSC should be suspected [44]. Whether there is an increased risk for malignant melanoma under TNFI has long been controversial without clear signals. An analysis of 11 European RA registries [45] and the most recent analysis of the Australian ARAD registry [46] for RA patients found no evidence of increased risk. Several studies found no significantly increased risk of progression from known cervical intraepithelial dyspalsia to cervical carcinoma with TNFI [47]. A recent analysis of U.S. insurance data suggests an increased risk of lymphoma [48], which was not found in studies from several national registries [49]. The important and reassuring data on studies of tumor recurrence risk with TNFI have been presented above. In this context, data from the field of immuno-oncological therapies with so-called checkpoint inhibitors (ICPI) are interesting: Mouse models [50,51] and a first case series [52] suggest that the combination of ICPI with TNFI might not only lead to a lower rate of immune-mediated side effects but also to better tumor control.
Rituximab: Data situation: very good.
Although rituximab has been used in Germany for a long time, following the old ACR recommendations relatively soon after curative tumor therapy, the literature on rheumatologic diseases (especially RA) does not contain any evidence of an increased risk for tumor recurrence or for new malignancies. Favorable registry data on tumor recurrence risk were presented above.
Tocilizumab, Sarilumab: Data situation: good for tocilizumab, limited for sarilumab.
Tocilizumab finds therapeutic use in various therapeutic situations in oncology: Treatment of Castleman’s disease (approval in Japan), Treatment of cytokine storm after CAR T-cell therapy (approval), Treatment of immune-mediated side effects of checkpoint inhibitors (case series, expert opinion) [53]. Mechanism of action considerations that cast tocilizumab in a favorable light in the context of malignancies should not be overinterpreted. A large analysis of US insurance databases found at least no higher risk of malignant events in RA with tocilizumab compared with TNFI or abatacept, with NMSC excluded from the analysis [54].
Abatacept: Data situation: good.
Again, considerations of the mechanism of action, which is exactly opposite to that of the checkpoint inhibitor ipilimumab, should not be overinterpreted. However, a large registry study [55] and an analysis of US insurance data [56] support an increased risk for NMSC even compared with other biologics. For overall cancer incidence and melanoma incidence, data are conflicting [57].
Secukinumab, Ixekizumab: Data: moderate for secukinumab, limited for ixekizumab.
IL17 signaling pathways can theoretically be both tumor-promoting and tumor-inhibiting. The reasoning of the German S3 guideline for the treatment of psoriasis, which gives preference to secukinumab and ustekinumab over TNFI in the case of malignant previous disease, does not appear conclusive with regard to secukinumab [58]. Data on the alleged increase in tumor risk with TNFI are overinterpreted here and contrasted with data on secukinumab, which show no evidence of increased cancer incidence but are also not suitable for investigating this question due to low case numbers and short follow-up.
Ustekinumab: Data situation: good.
In an evaluation of the PSOLAR registry of psoriasis therapy, ustekinumab and methotrexate demonstrated lower overall cancer risk compared with TNFi [59]. This would support the above-mentioned recommendation in the German psoriasis guideline, but should ideally be confirmed in other studies and therapy situations, especially since the aforementioned difficult-to-interpret data from FDA adverse event reports argue for a higher risk compared to apremilast.
Anakinra, canakinumab: Data: moderate to good.
Anakinra is used for the treatment of Schnitzler’s syndrome. In this disease, there is an association of urticarial vasculitis with monoclonal gammopathy of unclear significance, which is a precancerous condition. In the CANTOS trial, patients with elevated CRP after myocardial infarction received either canakinumab or placebo [60]. Despite meeting the primary endpoint, this study did not lead to approval in this indication, but there was a lower incidence of bronchial carcinoma in the study arm. Indeed, interleukin-1 may have tumor-promoting effects. This observation will be followed up in an oncology study program.
Belimumab: Data: moderate.
Separate findings on the risk for malignant diseases are not available.
JAK inhibitors: Data situation: good for tofacitinib, moderate for baricitinib, limited for upadacitinib.
The consideration of the tumor risk of JAK inhibitors should be particularly well-founded now and in the future, since these are highly potent immunosuppressive drugs and certain signals can already be observed, but they do not yet provide a clear picture. Patients with myeloproliferative neoplasms (MPN) have clustered monoclonal B-cell populations in the bone marrow and are therefore at higher risk for B-cell neoplasms. A hematooncology group from Vienna evaluated a cohort of MPN patients, some of whom were treated with different approved and experimental JAK inhibitors [61]. This cohort showed a significantly increased risk of aggressive B-cell neoplasms with JAK inhibitors. This observation was supported by a mouse model from the research group but was not confirmed by an analogous study of a larger MPN cohort from MD Anderson [62]. In the randomized RA trial program of upadacitinib, a significantly higher risk of NMSC was found at the unapproved dose of 30 mg than at 15 mg [63]. Also, with baricitinib, relatively more NMSCs occurred numerically at the higher dose 4 mg in RA than at 2 mg [64]. Meaningful registry data already exist only for tofacitinib. In the U.S. CORRONA registry, rates for NMSC and for cancer without NMSC were comparable with biologics under tofacitinib and did not differ significantly [65]. However, the possibility of bias in registers should be pointed out again. Also, this study is not yet fully published.
Therapy strategies depending on the situation
Against the background of these considerations, an attempt will be made in the following to formulate suggestions for the rheumatologic therapy of patients with a history of malignancy. These suggestions are a synthesis of existing recommendations in guidelines and the evidence described and reviewed. They were not subject to any consensus process and merely reflect the individual opinion of the author:
- Therapeutic decisions in this context are highly emotionally charged and must be discussed in detail with the patient and the oncology attending colleague.
- In a palliative therapy situation, a distinction must be made between whether the prognosis and oncological therapy options are very limited or whether there is the possibility of long-term disease control through (modern) oncological therapy concepts. In particular, the evaluation of DMARD therapies after immuno-oncologic therapies is difficult, but may be supported by oncologic experience for immunosuppressive therapy of immune-mediated side effects of these treatments. If the primary therapeutic goal is symptom relief, rheumatologic therapy can usually be pursued without restriction. The goal of long-term tumor control should be similar to curative therapy concepts.
- If the goal of therapy is curative, rheumatologic therapy can probably be given 5 years after completion of oncologic therapy without restriction. Multi-year oncologic maintenance therapies should not further delay this interval. Even beyond an interval of 5 years, abatacept, JAK inhibitors and perhaps TNF inhibitors should rather be avoided in malignant melanoma due to the unclear data, especially if alternative therapeutic options exist.
- In curatively treated lymphomas, one should be rather cautious with JAK inhibitors and perhaps also TNF inhibitors. In contrast, rituximab and perhaps tocilizumab have a beneficial effect in the presence of prior B-cell neoplasms or plasma cell dyscrasias.
- If the goal of therapy is curative, within the 5-year interval is recommended:
- Pausing basic therapy and bridging with glucocorticosteroids (or non-steroidal anti-inflammatory drugs) during chemotherapy.
- Consider interactions in long-term oncologic maintenance therapies.
- Otherwise, a consistent basic therapy in the sense of a graded concept, which is based on the individual tumor recurrence risk and the data available on the DMARDs in question. The data situation is to be evaluated both according to how many studies of what quality are available and according to whether evidence for lower or higher tumor risk has been shown in comparison with other substances.
- However, long-term uncontrolled inflammation or long-term high-dose glucocorticoid therapy due to excessive restraint before basic therapy should be avoided at all costs.
- Due to the high complexity of immune system-tumor interactions associated with immuno-oncologic therapies (checkpoint inhibitors), it is recommended that rheumatologic therapy decisions be made by a specialized center.
Take-Home Messages
- The decision on immunomodulatory or immunosuppressive therapies in patients with a history of malignancy must usually be made on an interdisciplinary basis and always in close consultation with the patient.
- An increasing wealth of data is helping to evaluate DMARD single agents. The data situation is complex to assess due to different limitations of the study formats, but with very few exceptions, the picture is rather positive.
- For a few substances (TNF inhibitors, rituximab), studies exist that have primarily investigated the risk of tumor recurrence after malignant disease has occurred. For other compounds, one can be informed by data on the risk of incident tumors and, to a limited extent, by looking at preclinical models.
- Only to cyclophosphamide in high cumulative dose is there a strong signal of increased tumor risk. With alternatives available, one should probably rather avoid abatacept, JAK inhibitors, and perhaps TNF inhibitors in some situations. For TNF inhibitors, the data situation is particularly conclusive in certain situations (e.g., condition after breast carcinoma) and appears rather favorable there.
- Several substances increase the risk of non-melanocytic skin cancers. This risk can be well addressed by initiating consistent skin tumor screening.
Literature:
- Baecklund E, Iliadou A, Askling J, et al: Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 2006; 54(3): 692-701.
- Zhang Y, Sun Y, Zhang Q: Prognostic value of the systemic immune-inflammation index in patients with breast cancer: a meta-analysis. Cancer Cell Int 2020; 20: 224.
- Hirano T, Hirayama D, Wagatsuma K, et al: Immunological Mechanisms in Inflammation-Associated Colon Carcinogenesis. Int J Mol Sci 2020; 21(9): 3062.
- Moik F, Zöchbauer-Müller S, Posch F, et al: Systemic Inflammation and Activation of Haemostasis Predict Poor Prognosis and Response to Chemotherapy in Patients with Advanced Lung Cancer. Cancers (Basel) 2020; 12(6): E1619.
- Kampan NC, Madondo MT, McNally OM, et al: Interleukin 6 Present in Inflammatory Ascites from Advanced Epithelial Ovarian Cancer Patients Promotes Tumor Necrosis Factor Receptor 2-Expressing Regulatory T Cells. Front Immunol 2017; 8: 1482.
- Krathen MS, Gottlieb AB, Mease PJ: Pharmacologic immunomodulation and cutaneous malignancy in rheumatoid arthritis, psoriasis, and psoriatic arthritis. J Rheumatol 2010; 37(11): 2205-2215.
- Mercer LK, Davies R, Galloway JB, et al: Risk of cancer in patients receiving non-biologic disease-modifying therapy for rheumatoid arthritis compared with the UK general population. Rheumatology 2013; 52: 9198.
- Nocturne G, Mariette X: Sjögren’s syndrome-associated lymphomas: an update on pathogenesis and management. Br J Haematol 2015; 168(3): 317-327.
- Feldman CH, Liu J, Feldman S, et al: Risk of high-grade cervical dysplasia and cervical cancer in women with systemic lupus erythematosus receiving immunosuppressive drugs. Lupus 2017 Jun; 26(7): 682-689.
- Lopez-Olivo MA, Colmegna I, Karpes Matusevich AR, et al: Systematic Review of Recommendations on the Use of Disease-Modifying Antirheumatic Drugs in Patients With Rheumatoid Arthritis and Cancer. Arthritis Care Res (Hoboken) 2020; 72(3): 309-318.
- Singh JA, Furst DE, Bharat A, et al: 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012; 64(5): 625-639.
- Singh JA, Saag KG, Bridges SL Jr, et al: 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2016 Jan; 68(1): 1-25.
- Penn I: Evaluation of transplant candidates with pre-existing malignancies. Ann Transplant 1997; 2(4): 14-17.
- Tarella C, Passera R, Magni M, et al: Risk factors for the development of secondary malignancy after high-dose chemotherapy and autograft, with or without rituximab: a 20-year retrospective follow-up study in patients with lymphoma. J Clin Oncol 2011 Mar 1; 29(7): 814-824.
- Strangfeld A, Hierse F, Rau R, et al: Risk of incident or recurrent malignancies among patients with rheumatoid arthritis exposed to biologic therapy in the German biologics register RABBIT. Arthritis Res Ther 2010; 12: R5.
- Dixon WG, Watson KD, Lunt M, et al: Influence of antitumor necrosis factor therapy on cancer incidence in patients with rheumatoid arthritis who have had a prior malignancy: results from the British Society for Rheumatology Biologics Register. Arthritis Care Res 2010; 62: 755-763.
- Pappas DA, Rebello S, Liu M, et al: Therapy with Biologic Agents After Diagnosis of Solid Malignancies: Results from the Corrona Registry. J Rheumatol 2019; 46(11): 1438-1444.
- Dreyer L, Cordtz RL, Hansen IMJ, et al: Risk of second malignant neoplasm and mortality in patients with rheumatoid arthritis treated with biological DMARDs: a Danish population-based cohort study. Ann Rheum Dis 2018; 77(4): 510-514.
- Raaschou P, Söderling J, Turesson C, et al: Tumor necrosis factor inhibitors and cancer recurrence in Swedish patients with rheumatoid arthritis: a nationwide population-based cohort study. Ann Intern Med 2018; 169: 291-299.
- Xie W, Xiao S, Huang Y, et al: A meta-analysis of biologic therapies on risk of new or recurrent cancer in patients with rheumatoid arthritis and a prior malignancy. Rheumatology (Oxford) 2019 Oct 17; [Epub ahead of print].
- Shelton E, Laharie D, Scott FI, et al: Cancer Recurrence Following Immune-Suppressive Therapies in Patients With Immune-Mediated Diseases: A Systematic Review and Meta-analysis. Gastroenterology 2016; 151(1): 97-109.e4.
- Mamtani R, Clark AS, Scott FI, et al: Association between breast cancer recurrence and immunosuppression in rheumatoid arthritis and inflammatory bowel disease: a cohort study. Arthritis Rheumatol 2016; 68: 2403-2411.
- Mercer LK, Lunt M, Low AL, et al: Risk of solid cancer in patients exposed to anti-tumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2015; 74(6): 1087-1093.
- Schmalzing M, Strangfeld A, Tony HP: Drug therapy of rheumatoid arthritis with a history of malignancy. Epidemiological data [Medication treatment of rheumatoid arthritis with a history of malignancy. Epidemiological data]. Z Rheumatol 2016; 75(1): 22-31.
- Regierer AC, Strangfeld A: Rheumatoid arthritis treatment in patients with a history of cancer. Curr Opin Rheumatol 2018; 30(3): 288-294.
- Shelton E, Laharie D, Scott FI, et al: Cancer Recurrence Following Immune-Suppressive Therapies in Patients With Immune-Mediated Diseases: A Systematic Review and Meta-analysis. Gastroenterology 2016; 151(1): 97-109.e4.
- De Cock D, Hyrich K: Malignancy and rheumatoid arthritis: epidemiology, risk factors and management. Best Pract Res Clin Rheumatol 2018; 32(6): 869-886.
- Lange E, Blizzard L, Venn A, et al: Disease-modifying anti-rheumatic drugs and non-melanoma skin cancer in inflammatory arthritis patients: a retrospective cohort study. Rheumatology (Oxford) 2016; 55(9): 1594-1600.
- Solomon DH, Glynn RJ, Karlson EW, et al: Adverse Effects of Low-Dose Methotrexate: A Randomized Trial. Ann Intern Med 2020 Feb 18; [Epub ahead of print].
- Rizzi R, Curci P, Delia M, et al: Spontaneous remission of “methotrexate-associated lymphoproliferative disorders” after discontinuation of immunosuppressive treatment for autoimmune disease. Review of the literature. Med Oncol 2009; 26(1): 1-9.
- Hellgren K, Baecklund E, Backlin C, et al: Rheumatoid Arthritis and Risk of Malignant Lymphoma: Is the Risk Still Increased? Arthritis Rheumatol 2017; 69(4): 700-708.
- Behrens F, Koehm M, Burkhardt H: Update 2011: leflunomide in rheumatoid arthritis – strengths and weaknesses. Curr Opin Rheumatol 2011; 23(3): 282-287.
- Ruiz-Irastorza G, Ugarte A, Egurbide MV,et al: Antimalarials may influence the risk of malignancy in systemic lupus erythematosus. Ann Rheum Dis 2007; 66(6): 815-817.
- Fardet L, Nazareth I, Petersen I: Effects of chronic exposure of hydroxychloroquine/chloroquine on the risk of cancer, metastasis, and death: a population-based cohort study on patients with connective tissue diseases. Clin Epidemiol 2017; 9: 545-554.
- Lopez A, Pouillon L, Beaugerie L, et al: Colorectal cancer prevention in patients with ulcerative colitis. Best Pract Res Clin Gastroenterol 2018; 32-33: 103-109.
- Moore TJ: Safety perspectives: cancer risks of biological products for psoriasis, www.ismp.org/quarterwatch/safety-perspectives (accessed: March 29, 2018).
- Thai A, Prindiville T: Hepatosplenic T-cell lymphoma and inflammatory bowel disease. J Crohns Colitis 2010; 4: 511-522.
- Ertz-Archambault N, Kosiorek H, Taylor GE, et al: Association of Therapy for Autoimmune Disease With Myelodysplastic Syndromes and Acute Myeloid Leukemia. JAMA Oncol 2017; 3(7): 936-943.
- Scott FI, Mamtani R, Brensinger CM, et al: Risk of Nonmelanoma Skin Cancer Associated With the Use of Immunosuppressant and Biologic Agents in Patients With a History of Autoimmune Disease and Nonmelanoma Skin Cancer. JAMA Dermatol 2016; 152(2): 164-172.
- Muellenhoff MW, Koo JY: Cyclosporine and skin cancer: an international dermatologic perspective over 25 years of experience. A comprehensive review and pursuit to define safe use of cyclosporine in dermatology. J Dermatolog Treat 2012; 23(4): 290-304.
- Vos M, Plasmeijer EI, van Bemmel BC, et al: Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant 2018; 37(7): 853-859.
- Hellbacher E, Hjorton K, Backlin C, et al: Malignant lymphoma in granulomatosis with polyangiitis: subtypes, clinical characteristics and prognosis. Acta Oncol 2019; 58(11): 1655-1659.
- Kang KY, Kim HO, Yoon HS, et al: Incidence of cancer among female patients with systemic lupus erythematosus in Korea. Clin Rheumatol 2010; 29(4): 381-388.
- Raaschou P, Simard JF, Asker Hagelberg C, et al: Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. BMJ 2016; 352: i262.
- Mercer LK, Askling J, Raaschou P, et al: Risk of invasive melanoma in patients with rheumatoid arthritis treated with biologics: results from a collaborative project of 11 European biologic registries. Ann Rheum Dis 2017; 76(2): 386-391.
- Staples MP, March L, Hill C, Lassere M, Buchbinder R: Malignancy risk in Australian rheumatoid arthritis patients treated with anti-tumour necrosis factor therapy: an update from the Australian Rheumatology Association Database (ARAD)prospective cohort study. BMC Rheumatol 2019; 3: 1.
- Cordtz R, Mellemkjær L, Glintborg B, et al: Malignant progression of precancerous lesions of the uterine cervix following biological DMARD therapy in patients with arthritis. Ann Rheum Dis 2015; 74(7): 1479-1480.
- Calip GS, Patel PR, Adimadhyam S, et al: Tumor necrosis factor-alpha inhibitors and risk of non-Hodgkin lymphoma in a cohort of adults with rheumatologic conditions. Int J Cancer 2018; 143(5): 1062-1071.
- Mercer LK, Galloway JB, Lunt M, et al: Risk of lymphoma in patients exposed to antitumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2017; 76(3): 497-503.
- Perez-Ruiz E, Minute L, Otano I, et al: Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 2019; 569(7756): 428-432.
- Bertrand F, Montfort A, Marcheteau E, et al: TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat Commun 2017; 8(1): 2256.
- Badran YR, Cohen JV, Brastianos PK,et al: Concurrent therapy with immune checkpoint inhibitors and TNFα blockade in patients with gastrointestinal immune-related adverse events. J Immunother Cancer 2019; 7(1): 226.
- Kim ST, Tayar J, Trinh VA, et al: Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis 2017; 76(12): 2061-2064.
- Kim SC, Pawar A, Desai RJ, et al: Risk of malignancy associated with use of tocilizumab versus other biologics in patients with rheumatoid arthritis: A multi-database cohort study. Semin Arthritis Rheum 2019; 49(2): 222-228.
- Wadström H, Frisell T, Askling J; Anti-Rheumatic Therapy in Sweden (ARTIS)Study Group: Malignant Neoplasms in Patients With Rheumatoid Arthritis Treated With Tumor Necrosis Factor Inhibitors, Tocilizumab, Abatacept, or Rituximab in Clinical Practice: A Nationwide Cohort Study From Sweden. JAMA Intern Med 2017; 177(11): 1605-1612.
- Montastruc F, Renoux C, Dell’Aniello S, et al: Abatacept initiation in rheumatoid arthritis and the risk of cancer: a population-based comparative cohort study. Rheumatology (Oxford) 2019; 58(4): 683-691.
- de Germay S, Bagheri H, Despas F, et al: Abatacept in rheumatoid arthritis and the risk of cancer: a world observational post-marketing study. Rheumatology (Oxford) 2019 Dec 27; [Epub ahead of print].
- Nast A, Amelunxen L, Augustin M, et al: S3 guideline on the therapy of psoriasis vulgaris update 2017. AWMF register no. 013/001.
- Fiorentino D, Ho V, Lebwohl MG, et al: Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol 2017; 77(5): 845-854.e5.
- Ridker PM, MacFadyen JG, Thuren T, et al: Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390(10105): 1833-1842.
- Porpaczy E, Tripolt S, Hoelbl-Kovacic A, et al: Aggressive B-cell lymphomas in patients with myelofibrosis receiving JAK1/2 inhibitor therapy. Blood 2018; 132(7): 694-706.
- Pemmaraju N, Kantarjian H, Nastoupil L, et al: Characteristics of patients with myeloproliferative neoplasms with lymphoma, with or without JAK inhibitor therapy. Blood 2019; 133(21): 2348-2351.
- Integrated safety summary, Abbvie data on file.
- Smolen JS, Genovese MC, Takeuchi T, et al: Safety Profile of Baricitinib in Patients with Active Rheumatoid Arthritis with over 2 Years Median Time in Treatment. J Rheumatol 2019; 46(1): 7-18.
- Kremer J, Bingham C, Cappelli L, et al: Comparison of Malignancy and Mortality Rates Between Tofacitinib and Biologic DMARDs in Clinical Practice: Five-Year Results from a US-Based Rheumatoid Arthritis Registry [abstract]. Arthritis Rheumatol 2019; 71 (suppl 10).
- Schmalzing M.: Rheumatologic drug therapy in case of malignoma history. Current Rheumatology 2020; Georg Thieme Verlag KG Stuttgart; doi: 10.1055/a-1247-4252.
InFo PAIN & GERIATURE 2020; 2(2): 6-11.