The increasing prevalence of heart failure is a major medical and economic problem. The number of patients hospitalized for heart failure has been increasing for years. The clinical picture of heart failure is complex. Typical symptoms include dyspnea, decreased performance, fatigue, and fluid retention.
The increasing prevalence of heart failure is a major medical and economic problem [1]. The number of patients hospitalized for heart failure has been increasing for years. The associated costs to the health care system are high [1,2].
Heart failure is defined as the inability of the heart to supply the body with sufficient blood and oxygen to maintain a stable metabolism under resting and exertional conditions. The clinical picture of heart failure is complex. Typical symptoms include dyspnea, decreased performance, fatigue, and fluid retention [1,2].
Current ESC recommendations differentiate between heart failure with reduced left ventricular ejection fraction (Heart Failure with reduced Ejection Fraction, HFrEF; EF <40%), with mildly impaired LV-EF (Heart Failure with mid-range Ejection Fraction, HFmrEF; EF 40-50%), and with preserved LV-EF (Heart Failure with preserved Ejection Fraction, HFpEF; LV-EF >50%) [1]. Each of these three heart failure categories is associated with a poor prognosis with increased morbidity and mortality [1,2].
Heart failure with reduced left ventricular ejection fraction (HFrEF)
Despite tremendous progress with a wide range of therapeutic options in pharmacology and device therapy (ICD, CRT) to interventional or surgical procedures (Mitra clip, VAD), the prognosis of patients with HFrEF remains poor [1]. During a median follow-up of 47 months, mortality was 32% in HFrEF patients with LV-EF of 35-50%. With an LV-EF <35%, mortality increased to 41% [1–3].
Cardiopulmonary exercise capacity, as measured by maximal oxygen uptake in spiroergometry (peak VO2 ml/min-1/kg-1), is markedly reduced in patients with HFrEF. It is lower in women than in men and decreases even more significantly with age and after decompensation [3].
Peak VO2 is considered a significant predictor of all-cause mortality [3], and a 1 ml/min-1/kg-1 lower peak VO2 is associated with a 16% higher all-cause mortality [3]. Causes of reduced exercise capacity in HFrEF include reduced left ventricular pump function, reduced pulmonary capacity caused by cardiac dysfunction, and marked peripheral skeletal muscle deconditioning with reduced muscle mass [1,3]. Sarcopenia (muscle wasting) exists in 30-50% of HFrEF patients [3]. Furthermore, a lack of heart rate adaptation (chronotropic incompetence) and inadequate peripheral muscle blood flow due to peripheral vasoconstriction in the presence of neuroendocrine and sympathetic overstimulation may further limit physical performance.
Heart failure with preserved left ventricular ejection fraction (HFpEF).
The pathophysiology of heart failure with preserved LV EF (HFpEF) is complex, heterogeneous, and not yet fully understood [2]. HFpEf is frequently associated with comorbidities such as diabetes mellitus and hypertension. The initial focus is on left ventricular filling dysfunction (diastolic dysfunction), usually with left ventricular hypertrophy and initially preserved pump function. Microvascular dysfunction can lead to myocardial damage and reduction in left ventricular systolic function as it progresses [2]. In addition to clinical symptoms (dyspnea, decreased performance, fluid retention), HFpEF is defined by an LV EF ≥50%, elevated natriuretic peptides (BNP >35 pg/ml, NT-proBNP >125 pg/ml), and echocardiographic evidence of structural heart disease (left ventricular hypertrophy, left atrial enlargement) or diastolic dysfunction [1,4]. The proportion of patients with HFpEF in the total heart failure population is assumed to be approximately 50%. The prognosis of these patients is comparably poor to those with HFrEF [1,4]. In contrast to HFrEF, pharmacologic therapy of patients with HFpEF is not assured. Morbidity and mortality of patients with HFpEF have not yet been reduced by pharmacotherapy similar to that used for HFrEF [1,4]. As a result, prevention and treatment of cardiovascular risk factors and comorbidities in HFpEF become proportionately even more important.
Patients with HFpEF tend to be older, more often female, more likely to have type 2 diabetes and/or hypertension, and have more comorbidities. These patients are often highly symptomatic, the physical resilience in everyday life is severely limited and the quality of life is thus significantly reduced [1,3,4], despite normal LV-EF! Recent studies show that heart failure in HFpEF patients is associated with comparable peripheral adaptation mechanisms as in patients with HFrEF, i.e., marked skeletal muscle deconditioning and reduced peripheral muscle mass [6].
In HFpEF patients, reduced exercise capacity is associated with significantly increased morbidity and mortality [3]. However, initial studies show that improvement in exercise capacity was also associated with a decrease in all-cause mortality [5]. Given this background and the unconfirmed pharmacotherapy, physical training is of paramount importance in the therapy of patients with HFpEF [1,4].
Physical activity and exercise in heart failure (HFrEF and HFpEF).
As a consequence of heart failure with exertional dyspnea, fatigue and exhaustion, the prevalence of prolonged physical inactivity is very high in this collective [3]. Individually tailored exercise interventions can effectively counteract skeletal muscle deconditioning with its negative effects on activities of daily living. In this way, regular physical activity can be highly effective in stabilizing physical performance and improving quality of life in patients with HFrEF and HFpEF [3].
For decades, physical training in the therapy of HFrEF patients has been investigated in many studies and evaluated in systematic reviews and meta-analyses. These consistently confirm the safety and high effectiveness of exercise interventions in HFrEF patients [3]. It is only in recent years that physical training in HFpEF patients has come into scientific focus. Studies similarly confirm the safety and effectiveness of adapted training in this patient group [3].
The results of these studies and meta-analyses demonstrate a significant improvement in quality of life as well as cardiopulmonary performance and mobility in everyday life (e.g., in the 6-minute walk test) [3]. Participation in exercise-based rehabilitation programs also significantly reduced hospitalization rates due to worsening heart failure. However, a reduction in mortality with exercise intervention has not been demonstrated in any of the current meta-analyses [3]. Therefore, current professional society guidelines recommend the use of individualized physical training for all clinically stable heart failure patients with HFrEF and HFpEF, and for any age group [1,3].
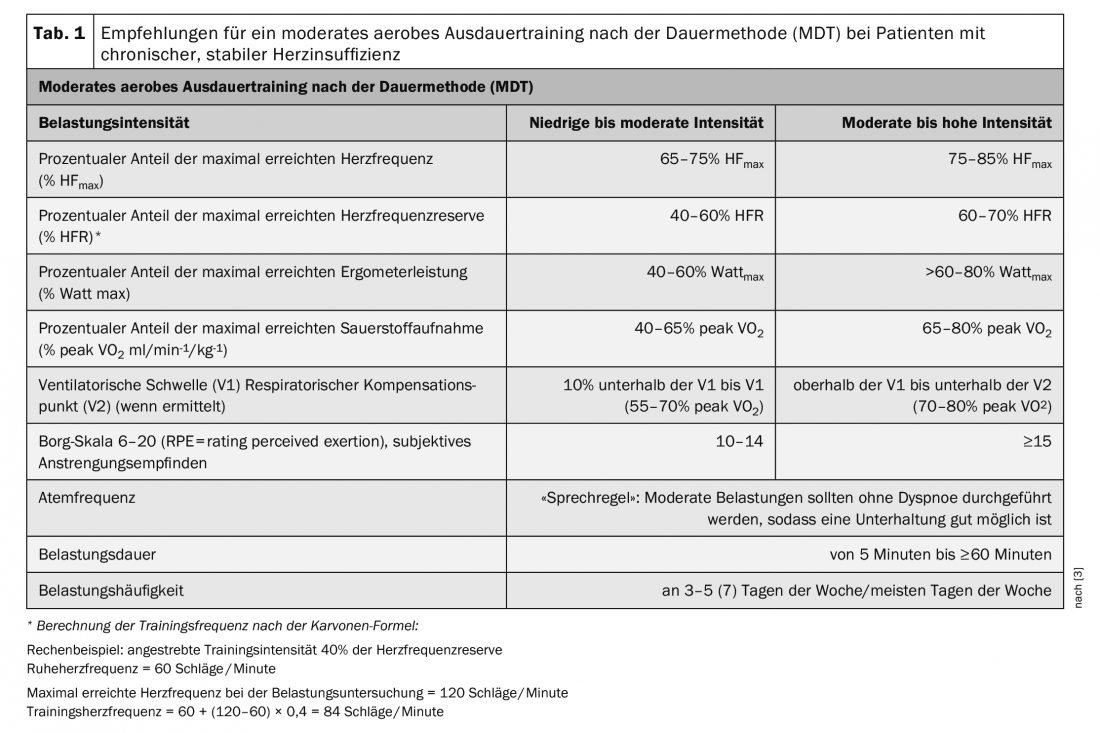
Patients with chronic stable heart failure are generally considered to be at increased risk. Therefore, prerequisites for starting an exercise intervention are clinical stability and optimal drug, device, or interventional therapy. Exercise-induced myocardial ischemia and arrhythmia as well as exsiccosis or hypervolemia must be ruled out before starting exercise [3]. Training recommendations should generally be based on thorough risk stratification including stress testing. The preferred exercise test is spiroergometry with submaximal exercise, if necessary in cooperation with an appropriate practice [3]. The results allow an assessment of the individual exercise tolerance and a prescription of adapted training forms and intensities. The training recommendations established in guidelines (Tables 1-3) provide “corridors” for lower and upper limits between which training is safe and effective. Individual training recommendations should be within these limits.
Training should be started in structured programs guided by the specialist therapist and monitored by a physician. All training measures should be continued in the long term, e.g. in outpatient heart failure groups. Clinically stable and practiced patients can also complete the training at home. As part of the guided exercise programs, the patient should be informed about the importance of regular physical activity on the course of the disease and receive advice on how to be more active in everyday life and leisure activities. Initial monitoring and supervision of training by professionals is important so that patients can properly assess their symptoms that may occur during training and recognize their relevance for continuing training. In this way, patients learn to realistically assess their resilience and their stress limits in everyday life as well [3].
Moderate aerobic endurance training according to the endurance method (MDT).
The best studied effects are those of moderate aerobic endurance training using the duration method (MDT). This form of training influences the progression and symptomatology of HFrEF in many ways. Well-documented effects include a positive effect on cardiac autonomic function with reduction in sympathetic activation, improvement in endothelial function, reduction in cardiac afterload, improvement in LV EF, reduction in left ventricular size, and improvement in skeletal muscle oxidative capacity [3]. The results of the meta-analyses confirm the significant improvement in cardiopulmonary exercise capacity (+2.82 to +3.10 ml/min-1/kg-1) with aerobic endurance training. Higher training intensities are associated with greater improvements [3]. Results of a recently published study provide evidence for the efficacy of exercise training in HFrEF patients independent of cardiopulmonary fitness at exercise initiation [3]. This means that even the weakest patients benefit! A significant increase in peak VO2 from exercise training was associated with an 81% risk reduction for the primary endpoint of hospital admission and/or mortality in patients with a high baseline peak VO2 and a 59% risk reduction in patients with a low baseline VO2 [3]. These results support the relevance of increasing a peak VO2 through adapted training in HFrEF patients.
In patients with HFpEF, the results of a recently published meta-analysis of studies on the efficacy of an MDT show a significant increase in cardiopulmonary performance (+1.67 ml/min-1/kg-1), mobility (6MWD: +33.9 m), and quality of life. In contrast, no positive effect on diastolic function or LV EF has been demonstrated by MDT [7].
Moderate aerobic endurance training according to the duration method (MDT) is therefore recommended as basic training for all patient groups. After the stress test on the bicycle ergometer, the maximum power (Wattmax) and heart rate (HRmax) achieved can be used to determine the individual training load. A percentage of HRmax or heart rate reserve (HFR) is given as a training recommendation. Exercise control as a percentage of HFR is recommended for chronotropic incompetence and beta-receptor blocker therapy, if appropriate. The indication in percent of Wattmax is helpful for patients in whom HR cannot be applied for training control, e.g. in atrial fibrillation. As a support or alternative, the subjective feeling of exertion via the Borg scale as well as the respiratory rate can be used for load control [3] (Tab. 1).
After spiroergometric testing, the training recommendation can be made as a percentage of peak VO2. Determination of ventilatory thresholds (VT) and respiratory compensation point (VT2) allow objective assessment of aerobic performance and more targeted training management [3].
Aerobic endurance training should be included at baseline in the form of short bouts of exercise (5-10 minutes ≥2 times/week) at low to moderate intensity (40-50% peak VO2, 40% HFR, Borg scale 10). If exercise tolerance is good, it is recommended to first increase exercise frequency (≥5 times/week, preferably daily) and increase exercise duration (20-30 minutes). This cautious approach is very important, especially for deconditioned and poorly resilient patients at the beginning of training. Patients with good exercise tolerance should be gradually introduced to more intense endurance exercise on an individual basis [3] (Table 1).
Endurance training according to the interval method
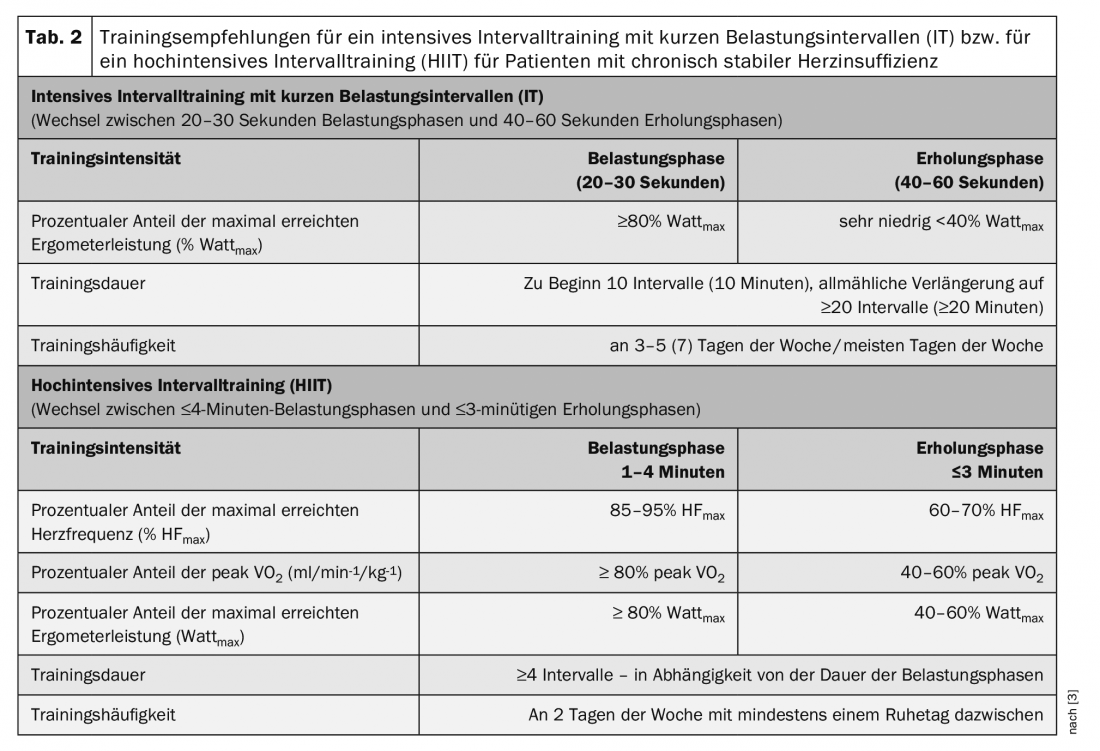
Training according to the interval method is characterized by a constant alternation of short load and recovery phases. This form of training makes it possible to repeatedly maintain a higher to very high intensity during the load phases. By now, the short-term efficacy and safety of interval training in heart failure patients is well studied. The focus of scientific discussion is on intense to high-intensity interval training. This is defined as repeated short (≤45 s) to long (2-4 min) bouts of exercise at high to very high, but not maximal, intensity (≥90% peak VO2) alternating with recovery bouts at moderate to low intensity.
Interval training with short intensive load phases (IT)
Interval training with short periods of intense exertion (20-30 seconds) alternating with recovery periods (40-60 seconds) (IT) twice as long ( Table 2) leads to comparable improvements in cardiopulmonary performance and mobility as MDT in HFrEF patients [3].
Such interval training is usually very well tolerated by patients with chronic stable heart failure and is considered suitable and safe for all patient groups. It is routinely used in many cardiac rehabilitation programs. It can be performed as an alternative or complementary to MDT. The use of IT is particularly recommended in elderly and/or particularly deconditioned and muscularly poorly resilient patients, as well as in patients suffering from comorbidities such as peripheral circulatory disorders of the legs and/or chronic obstructive pulmonary disease [3]. An IT should be used whenever patients cannot tolerate several minutes of continuous exercise (dyspnea or muscular).
Interval training with long periods of high-intensity exercise (HIIT)
A protocol with long loading phases that is frequently used is the so-called 4×4 protocol. After a short warm-up phase with moderate intensity (60% HRmax), four 4-minute load intervals (85-95% HRmax) alternate with 3-minute recovery phases (60-70% HRmax). The results on the effectiveness and suitability of this high-intensity interval training (HIIT) for HFrEF patients are still controversial. Results of meta-analyses do show greater effectiveness of HIIT for improving peak VO2 compared with MDT (+0.73 to +2.13 ml/min-1/kg-1). However, this superiority of HIIT could not be confirmed in a large multicenter prospective RCT [8]. The results of this study demonstrate comparable effectiveness of HIIT and MDT with significant increase in peak VO2 (HIIT +1.4 vs. MDT +1.8 ml/min-1/kg-1), with no significant differences between the two training groups. Positive influences on LV-EF or LV size by HIIT were not shown [8]. During the training intervention, adverse events were equally distributed in both groups. However, during the 52-week follow-up period, there was a nonsignificant trend toward more nonfatal and fatal adverse events in the HIIT group [8]. In addition, several authors report problems for many patients to reach the targeted very high exercise intensity during HIIT or to maintain it during the exercise phase.
The results of this study have led to the fact that high-intensity interval training (HIIT) is currently not fully recommended for HFrEF patients. This form of training should rather be considered in the long-term course of training therapy in stable patients with good exercise tolerance, possibly in addition to MDT, or HIIT should be performed alternatively with shorter and possibly less intense exercise phases [3] (Tab. 2).
For patients with HFpEF, a recently published meta-analysis demonstrated the safety, good tolerance, and efficacy of HIIT to increase peak VO2. Whether this training method is more effective in improving peak VO2 compared with MDT is still unclear. When isocaloric training protocols are compared in one study, the effectiveness of both methods is comparable [9].
In summary, the interval training method offers many design options. It can be adapted to individual needs via the duration and intensity of the load and recovery intervals as well as the ratio between load and recovery [3]. For all endurance training protocols, training on a bicycle ergometer (preferably with ECG monitoring) offers the advantage of body weight relief, exact dosability and reproducibility of the load. As an alternative or complement to training on a bicycle ergometer, aerobic endurance training can be performed in the form of walking and/or walking (brisk walking with increased use of arms) or walking with the use of poles (Nordic walking) on a firm level path, cross-trainer or on a treadmill [3].
Dynamic strength training
The benefits and safety of moderate dynamic strength training in heart failure patients have been well studied. Strength training has no negative effect on systolic heart function. The central hemodynamic response to moderate dynamic force loading is comparable to that during aerobic endurance training [3].
As a stand-alone form of exercise, strength training in HFrEF patients leads to improved muscular strength and counteracts the disease-related loss of muscle mass. It also has a positive impact on mobility, cardiopulmonary performance, and quality of life [3]. In addition, dynamic strength training is suitable as fall prevention.
In patients with HFrEF, the combination of strength and endurance training results in greater increases in cardiopulmonary exercise capacity (+2.48 ml/min-1/kg-1) and mobility (+50.05 m) compared with endurance training alone [3].
In HFpEF patients, 3 months of combined aerobic endurance training (MDT: 50-70% peak VO2) combined with moderate dynamic strength training (50-60% “1-repetition maximum” [1RM]) produced a significant increase in peak VO2 (+3.3 ml/min-1/kg-1) and improvement in echocardiographically determined diastolic function compared with a nonactive control group [10].
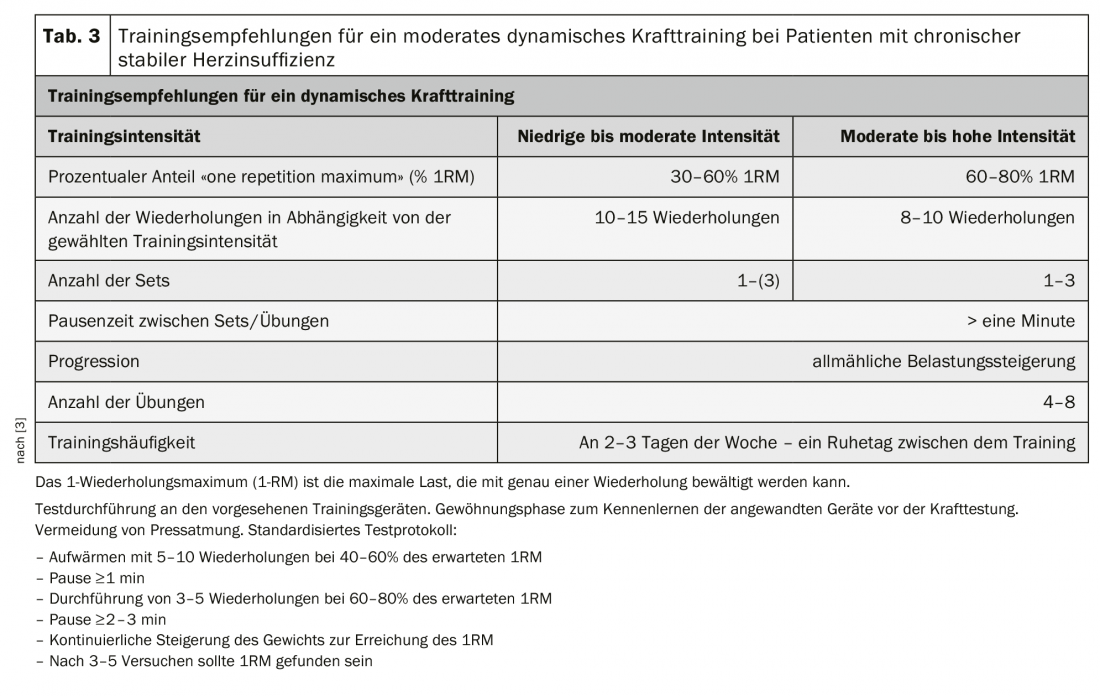
Dynamic strength training with low to moderate intensity and a low isometric component is therefore recommended as a complement to aerobic endurance training in both HFrEF and HFpEF patients [3]. A gentle introduction to strength training with very low intensity, low number of repetitions and slow pace of movement is used to learn and practice proper exercise execution and breathing. Press breathing with closed lips must be avoided at all costs. Patients should breathe in and out slowly with their mouths open in rhythm with the movement. Strength training should be incorporated with low to moderate loads <30-50 1RM. With good exercise tolerance, the intensity can be gradually increased on an individual basis over the course of the training program [3] (Table 3).
Literature:
- Ponikowski P, Voors AA, Anker SD, et al: 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891-975.
- German Medical Association (BÄK), National Association of Statutory Health Insurance Physicians (KBV), Association of the Scientific Medical Societies (AWMF). National Health Care Guideline Chronic Heart Failure – Long Version, 3rd ed. Version 2. 2019 [cited: 17-06-2020]; doi: 10.6101/AZQ/000467. www.herzinsuffizienz.versorgungsleitlinien.de.
- S3 – Guideline on cardiac rehabilitation (LL-KardReha) in German-speaking Europe, Germany, Austria, Switzerland (D-A-CH), long version – part 1, 2019; AWMF registry number: 133/001, www.awmf.org.
- Tschöpe C, Birner C, Böhm M, et al: Heart failure with preserved ejection fraction: current management and future strategies: Expert opinion on the behalf of the Nucleus of the “Heart Failure Working Group” of the German Society of Cardiology (DKG). Clin Res Cardiol 2018; 107: 1-19.
- Orimoloye OA, Kambhampati S, Hicks AJ, et al: Higher cardiorespiratory fitness predicts long-term survival in patients with heart failure and preserved ejection fraction: the Henry Ford Exercise Testing (FIT) Project. Arch Med Sci 2019; 15: 350-358.
- Tucker WJ, Haykowsky MJ, Seo Y, et al: Impaired Exercise Tolerance in Heart Failure: Role of Skeletal Muscle Morphology and Function. Curr Heart Fail Rep 2018; 15: 323-331.
- Fukuta H, Goto T, Wakami K, et al: Effects of exercise training on cardiac function, exercise capacity, and quality of life in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Heart Fail Rev 2019; 24: 535-547.
- Ellingsen Ø, Hall M, Conraads V, et al: High intensity interval training in heart failure patients with reduced ejection fraction. Circulation 2017; 135: 839-849.
- Gomes Neto M, Durães AR, Conceição LSR, et al: High intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure with reduced ejection fraction: A systematic review and meta-analysis. Int J Cardiol 2018; 261: 134-141.
- Edelmann F, Gelbrich G, Düngen HD, et al: Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol 2011; 58: 1780-1791.
HAUSARZT PRAXIS 2020; 15(9): 11-16