In addition to history, clinical examination and imaging, the determination of antibodies is an important method for the diagnosis of dermatomyositis as well as a prognostic clue. Several specific antibodies are exclusive to dermatomyositis and correlate with specific clinical manifestations and courses. Various immunomodulating agents are available for treatment, which can also be used in combination depending on the individual clinical picture.
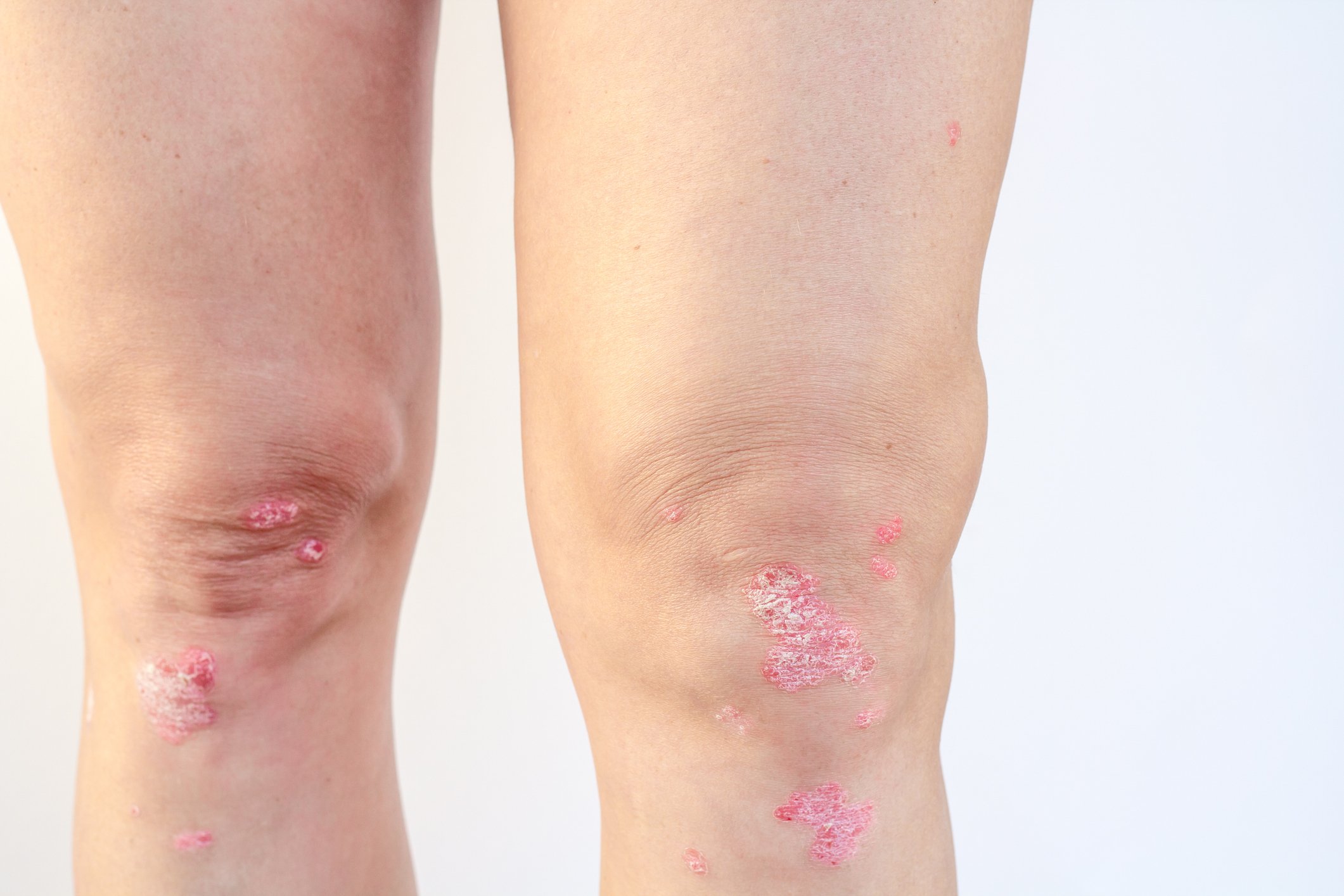
Dermatomyositis is an inflammatory autoimmune systemic disease belonging to the group of collagenoses and is one of the rare diseases. The spectrum of symptoms is broad and can include muscle, skin, vascular, lung and joint involvement. There is also an increased risk of tumors. Anamnestic evidence includes proximally emphasized muscle weakness and myalgias in addition to signs of collagenosis. Clinical symptoms include skin or other organ manifestations and declining muscle strength according to the British Medical Research Council (BMRC). Imaging techniques can provide informative information on differential diagnostic aspects as well as on the extent of disease (localization/number of affected muscle groups), but an inconspicuous MRI or EMG finding does not exclude myositis (cave!). Muscle or skin biopsy is still considered the diagnostic gold standard, but autoantibody measurement is becoming increasingly important in dermatomyositis, explained PD Britta Maurer, MD, Department of Rheumatology, University Hospital Zurich [1,2]. However, it is important to note that a negative antinuclear antibody (ANA) finding does not rule out MDA-5-positive dermatomyositis, which has a cytoplasmic immunofluorescence pattern.
High diagnostic and progression relevance
The corresponding specific antibodies occur exclusively in dermatomyositis, designate specific clinical subtypes, and allow estimation of organ involvement, prognosis, and response to therapy. Which autoantibodies should be measured is summarized in overview 1. Antibody findings correlate with clinical courses. MI-2 is most often associated with a mild course and MDA-5 with a progressive course and pulmonary involvement. NXP-2 and TIF-1-gamma are associated with malignancy (especially TIF-1-gamma in adults). Screening for immunofluorescence patterns reveals an AC4 pattern. This can be correlated with immunoblot and ELISA results for confirmation, increasing the specificity of the finding by reducing the risk of a false-positive result, the speaker explained. Other diagnostic steps to detect muscle involvement include MRI and EMG, which also serve as guidance for muscle biopsy. MRI has the advantage over EMG in allowing differentiation between acute versus degenerative stages. This has implications for therapeutic options: immunomodulatory therapy is more likely to be indicated for symptoms of acute muscle weakness, whereas physiotherapeutic measures are more likely to be prescribed for indications of a degenerative stage. The EMG allows a differential diagnostic differentiation from neuropathogenic diseases, especially myasthenia gravis or even motor neuron diseases. However, as mentioned at the outset, an unremarkable MRI or EMG finding does not rule out dermatomyositis. Multifocal findings can be detected by MRI, unlike EMG, but not all primary perivascular inflammation and reduced muscle perfusion due to small vessel damage.
| Dermatomyosistis: increased risk of malignancy Tumor screening should be performed for any newly diagnosed dermatomyositis as well as for refractory disease. That there is an association between dermatomyositis and malignancy has been empirically demonstrated, although data on prevalence and type of tumors vary across studies [3]. CT of the chest, abdomen, and pelvis has been shown to have high sensitivity for the detection of carcinogenic changes [3]. In addition, colonoscopy, mammography in women, cervical cancer screening, and prostate examination in men are suggested [1] . Solid tumors are more common than hematologic tumors. Temporally, cancer may occur before, concurrently with, or after the diagnosis of dermatomyositis, and the risk of tumor is increased over a 5-year period [1]. |
In the absence of specific autoantibodies or failure to respond to therapy, a muscle biopsy may be informative. Typical findings are mononuclear inflammatory infiltrates with CD4+ cells, but also macrophages and B cells with perivascular and perimysial dominance. Also common is loss of capillaries as well as MHC I upregulation on the sarcolemma of muscle fibers and MAK deposits in the area of the capillaries.
Immunomodulatory (combination) therapy recommended
The treatment regimen for first-line, second-line, and third-line therapy is shown in Table 1 [1,4]. Glucocorticoids are often used for induction therapy, and other conventional immunosuppressants for skin and muscle involvement are also used depending on the clinical presentation and severity of the disease. For pulmonary involvement, consider mycophenolate mofetil or azathioprine as first-line therapy. Intravenous immunoglobulins, rituximab, or cyclophosphamide may be used if there is no response to conventional basic therapeutics. Other options also include IL1 or IL6 inhibitors, T-cell co-stimulation inhibition, or increasingly JAK inhibitors. Depending on the patient’s condition, a combination of several different agents may be effective, as the speaker illustrated with a case study of a patient with rapidly progressive interstitial lung disease who was treated with a combination of hydroxychloroquine, rituximab and mycophenolate mofetil to induce remission [1].
Other symptoms in this patient included myopathy, arthritis, photosensitivity, and organic microangiopathy.
Source: ZDFT 2020
Literature:
- Maurer B: Dermatomyositis. PD Dr. med. Britta Maurer, Zürcher Dermatologische Fortbildungstage (ZDFT), 14./15.05.2020.
- Wolstencroft PW, Fiorentino DF: Curr Rheumatol Rep 2018; 20(5): 28.
- Hayley Leatham BS, et al: Medicine (Baltimore) 2018; 97(2): e9639.
- Oddis CV, Aggarwal R: Treatment in myositis. Nat Rev Rheumatol 2018; doi: 10.1038/nrrheum.2018.42
DERMATOLOGIE PRAXIS 2020; 30(4): 38-39 (published 8/25/20, ahead of print).