Topical pharmacotherapies offer the unique advantage that the products are applied directly to the diseased skin, resulting in local, distinctly direct contact between the product and the affected skin. The choice of vehicle depends on the type of dermatosis. Vehicle polarity and vehicle viscosity result in optimal contact between the vehicle and affected skin, allowing uncomplicated application and distribution of the product.
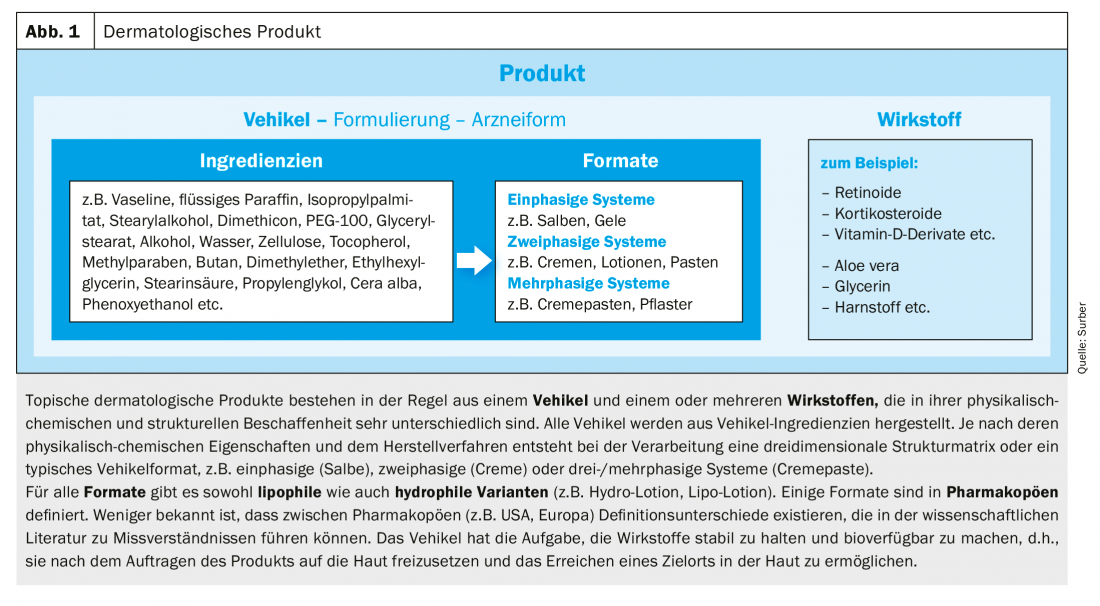
Topical therapies offer the unique advantage that the product is applied directly to the diseased skin. This leads – in contrast to all other therapies – to a local, distinctly direct contact between product and affected skin. For this reason, the ingredients that form the vehicle play a crucial role in the release and absorption of the active ingredients (Fig. 1) . The ingredients (sometimes also the active ingredients) are able to temporarily reduce the barrier function of the skin by various mechanisms. In addition, some drug-free vehicles also show pronounced clinical effects. Commonly used vehicle ingredients such as petrolatum, glycerin or dimethicone have efficacy in repairing the skin barrier. In addition, they contribute to the relief of symptoms in corticosteroid-sensitive skin diseases. Vehicle ingredients of topical products have intrinsic effects that can be clinically significant.
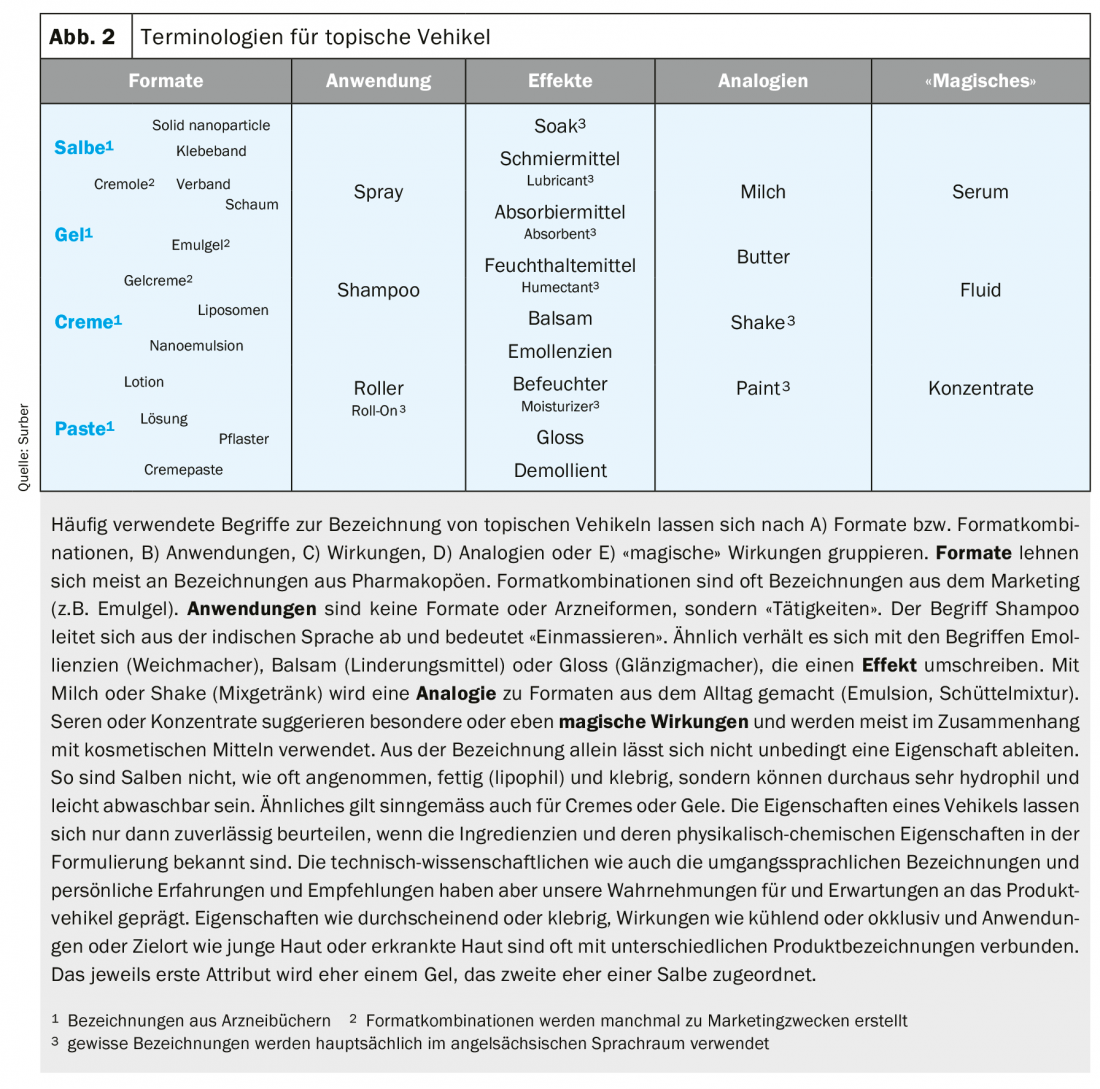
Vehicle Terminologies
A rich vocabulary has developed that is used by regulators, industry, scientists, health professionals, marketing people, patients and consumers to describe the vehicle of topical products. Vehicle formats are often associated with conveying specific effects (Fig. 2).
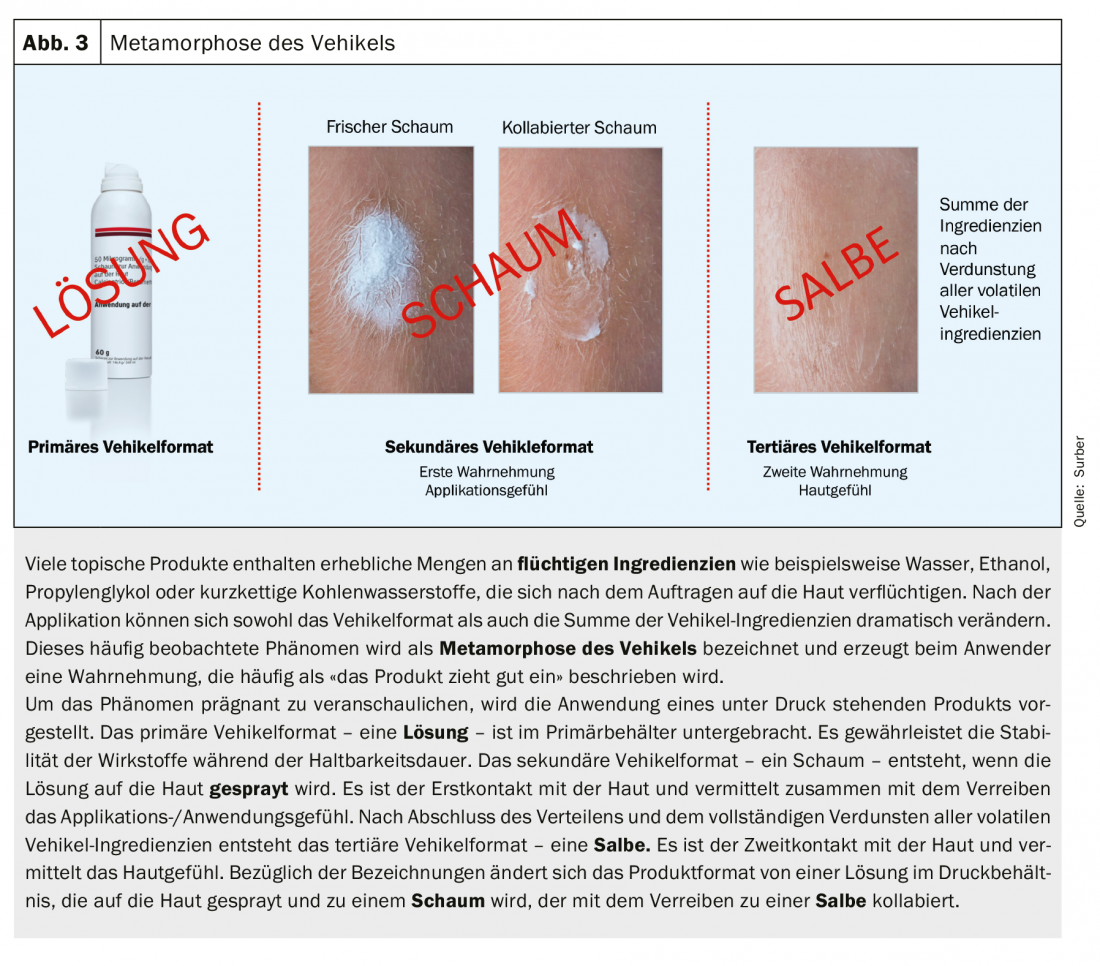
Metamorphosis of the vehicle
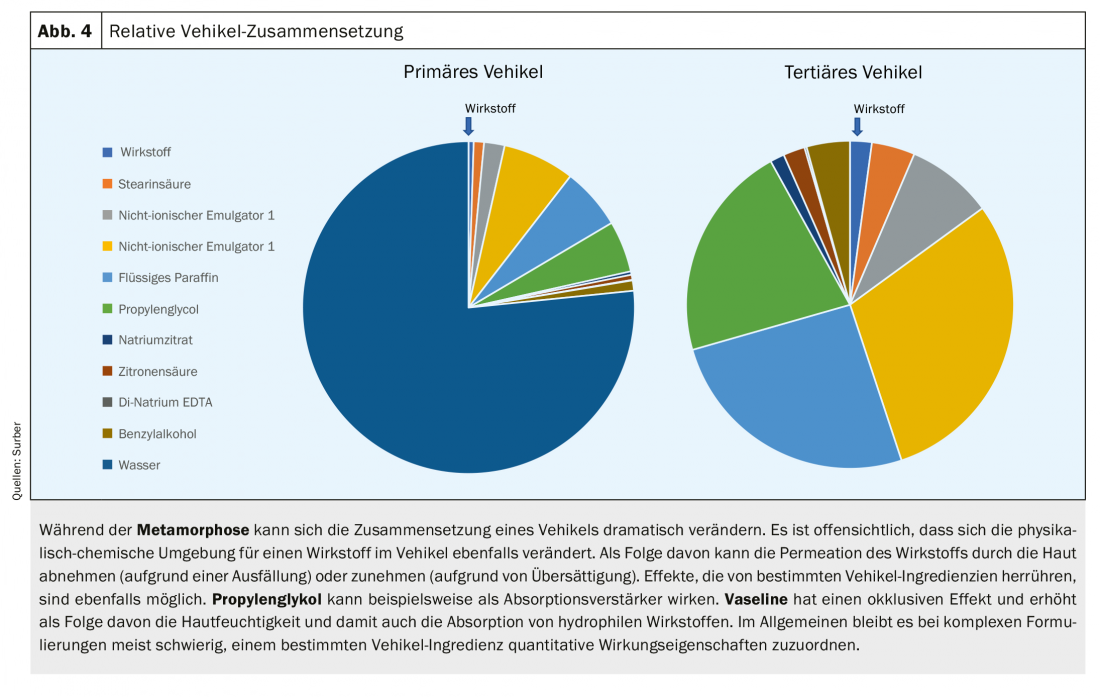
Vehicle formats (e.g., gels, creams) of topical products containing volatile vehicle ingredients can change dramatically after application to the skin ( Figs. 3 and 4) . The vehicle on the skin after application can be very different from the vehicle in the primary container (e.g., tube, bottle). This phenomenon of vehicle change is called metamorphosis of the vehicle. This phenomenon is often described by the patient or consumer as “the product absorbs well”. The metaphor “absorb” does not describe the absorption of substances into the skin, but the volatilization of volatile components, which is perceived as a change in the skin aspect. The vehicle format in the primary container should in no way be associated with a clinical effect. The paradigm – widely used with topical corticosteroids – that ointments are more effective than creams and creams are more effective than lotions is not valid. The effect of a vehicle can be explained primarily by the vehicle ingredients used.
Vehicle selection
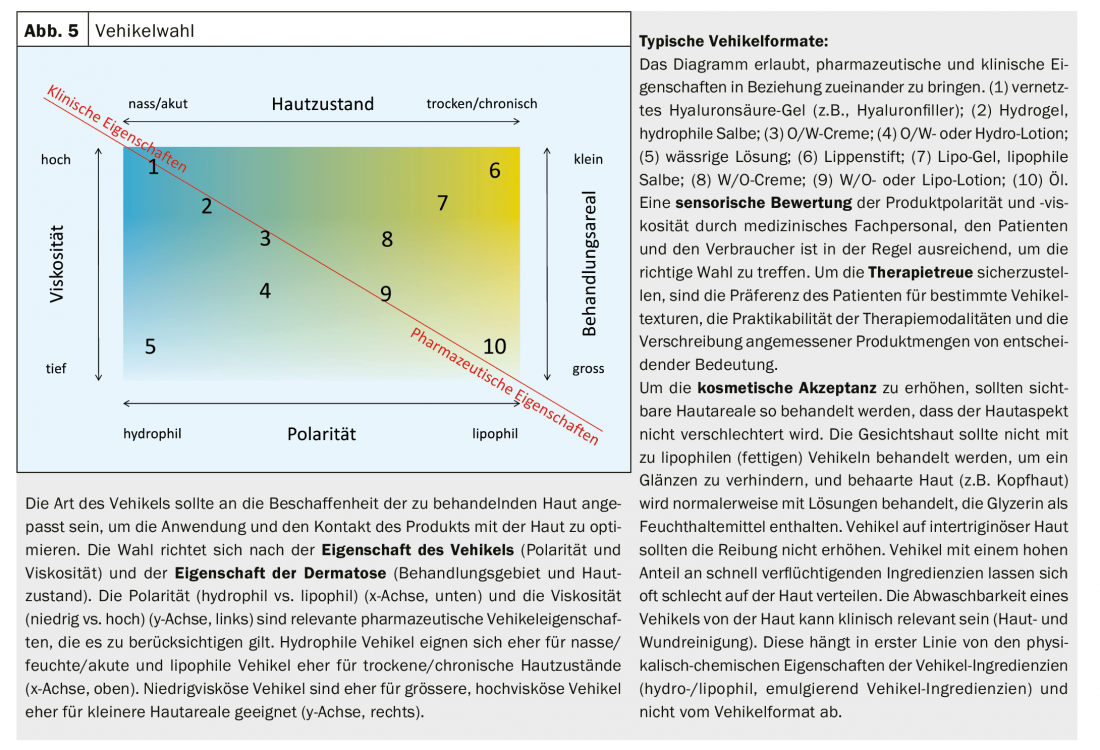
Adjusted vehicle polarity and viscosity result in optimal contact between vehicle and affected skin and allow uncomplicated application and distribution on the skin (Fig. 5).
Practical aspects
When prescribing topical products, explicit information on the amount to be applied per area and time should be provided whenever possible. An acceptable application amount for the patient is rarely more than 5 mg/cm2. For semi-solid vehicles, the “Finger Tip Unit” (400-500 mg) and for more liquid vehicles, a dosing aid with dosing tables and instructions have proven effective (e.g. pump with weight information per pump stroke). The frequency of administration and duration of treatment depends on the success of the treatment and is usually determined on an individual basis.
Typical therapeutic modalities have been developed to avoid or minimize adverse effects (corticosteroids, retinoids). Patients apply the topical drug daily only until symptoms improve (corticosteroids) or irritation occurs (retinoids) and then reduce the frequency of application. On days without drug treatment, products without active ingredient are recommended (interval therapy).
Localized and systemic adverse effects may occur with topical pharmacotherapy. Almost any component of a topical product can sensitize or irritate; examples include vehicle ingredients (propylene glycol), preservatives, fragrances, or active ingredients (corticosteroids). Patients with chronic wounds (e.g., incontinence-associated dermatoses) appear to be particularly susceptible. Extensive, frequent, and/or long-term use of topical products containing low-molecular-weight active ingredients (salicylic acid, crotamiton, imiquimod) may result in systemic absorption of the active ingredients. Due to a different ratio of body weight to body surface area, this risk is increased in newborns, infants, and children (e.g., treatment of scabies).
Three product categories
Topical dermatological products are offered in three different regulatory product categories. Pharmaceuticals and medical devices are used for diseased skin and cosmetics are used to protect and maintain the good condition of the skin. For drugs and medical devices, disease-related claims are allowed (e.g., atopic dermatitis), while this is not allowed for cosmetics. The effect of drugs is based on pharmacological and immunological principles, while the effect of medical devices is based on non-pharmacological and non-immunological principles. The distinction between diseased and non-diseased skin is fluid (xerotic vs. dry skin). Accordingly, all three product categories are offered and used for sometimes little different skin conditions. Terms such as “cosmeceutical” – neologisms of cosmetics and pharmaceuticals -, “cosmeceutical active” – analogous to the regulatory term “active pharmaceutical ingredient” – or claims such as “atopy-prone skin” are used to enhance certain products in the cosmetics category. This is sometimes a source of confusion, debate and regulatory complaints.
Take-Home Messages
- Topical pharmacotherapies offer the unique advantage that the products are applied directly to the diseased skin, resulting in local, distinctly direct contact between the product and the affected skin.
- The choice of vehicle depends on the type of dermatosis: size, localization and skin condition.
- Vehicle polarity (hydrophilic vehicles on hydrophilic (moist) skin, lipophilic vehicles on dry skin) and vehicle viscosity (low viscosity vehicles on large and high viscosity vehicles on small skin areas) result in optimal contact between vehicle and affected skin and allow uncomplicated application and distribution of the product.
- A realistic application amount is rarely more than 5 mg/cm2.
- For semi-solid vehicles the “Finger Tip Unit” (400-500 mg) is suitable, for more liquid vehicles mechanical dosing aids (e.g. pump with weights per pump stroke) with dosing tables/instructions.
Further reading:
- Surber C, Schmid-Grendelmeier P: General Principles of Topical Therapy of the Skin. In Brockow K, Moritz CG. (eds): Global Atlas of Skin Allergy. European Academy of Allergy and Clinical Immunology 2019; 226-229.
- Surber C, Knie U.: Metamorphosis of vehicles: mechanisms and opportunities. Curr Probl Dermatol 2018; 54: 152-165.
- European Pharmacopoeia 9.0 (1.1.2017), semi-solid preparations for cutaneous application.
- USP 41 (May 1, 2019), <1151> pharmaceutical dosage forms.
DERMATOLOGY PRACTICE 2020; 30(4): 12-16