Successful stroke treatment is carried out in dedicated centers (stroke units and stroke centers) by an experienced and specialized team. The standard of care in the acute phase is systemic thrombolysis and, in the case of occlusion of a proximal vessel, endovascular thrombectomy. The success of the treatment is strongly time-dependent. Therefore, early recognition of stroke symptoms and rapid referral to a hospital with appropriate expertise in acute treatment are essential.
Ischemic stroke is characterized by the acute onset of a focal neurologic deficit due to circumscribed reduced blood flow to the brain. With approximately 150 events per 100,000 population per year, it is the third leading cause of death in industrialized countries and the most common cause of permanent disability in adulthood. Nearly half of survivors remain disabled and/or in need of care. Emergency treatment of patients with stroke has been shown to improve survival and reduce disability and incapacity.
Pathophysiology: penumbra/infarct core
Structural metabolism is necessary to maintain cell structure. If this is not achieved, irreversible damage to the cell occurs. In addition, functional metabolism provides energy for the active activity of neuronal function. If the ischemic threshold is not reached, the functional metabolism fails with disturbance of electrical neuronal functions and clinical symptoms. The dysfunction is primarily reversible if normal blood flow is rapidly restored. Normally, the circulatory disturbance is more pronounced in the center (infarct core) than in the peripheral zone (penumbra), where a residual amount of blood flows via collaterals. Over time, there is a gradual enlargement of the infarct core at the expense of the penumbra. The speed of this process is highly variable and depends above all on the collateralization.
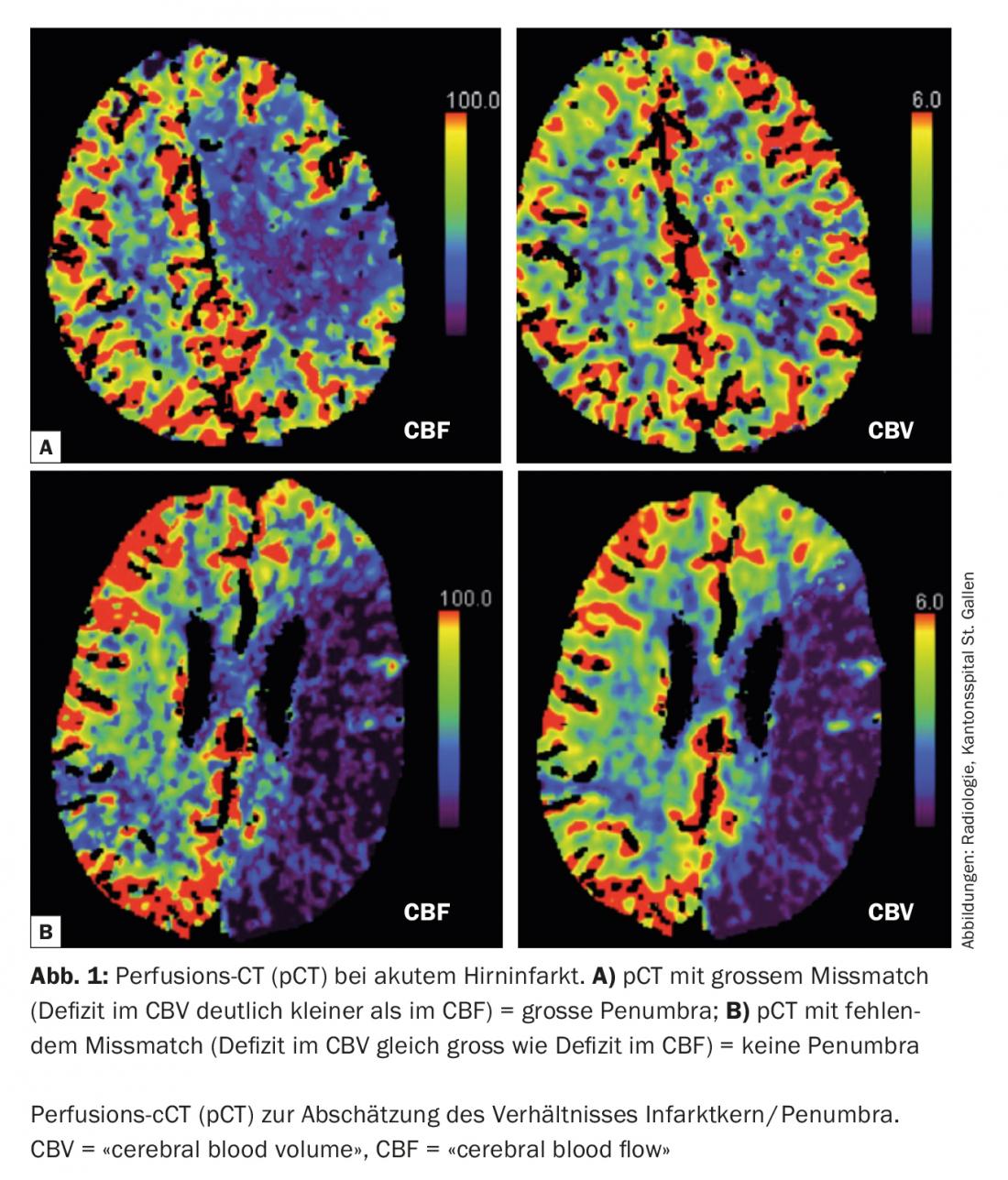
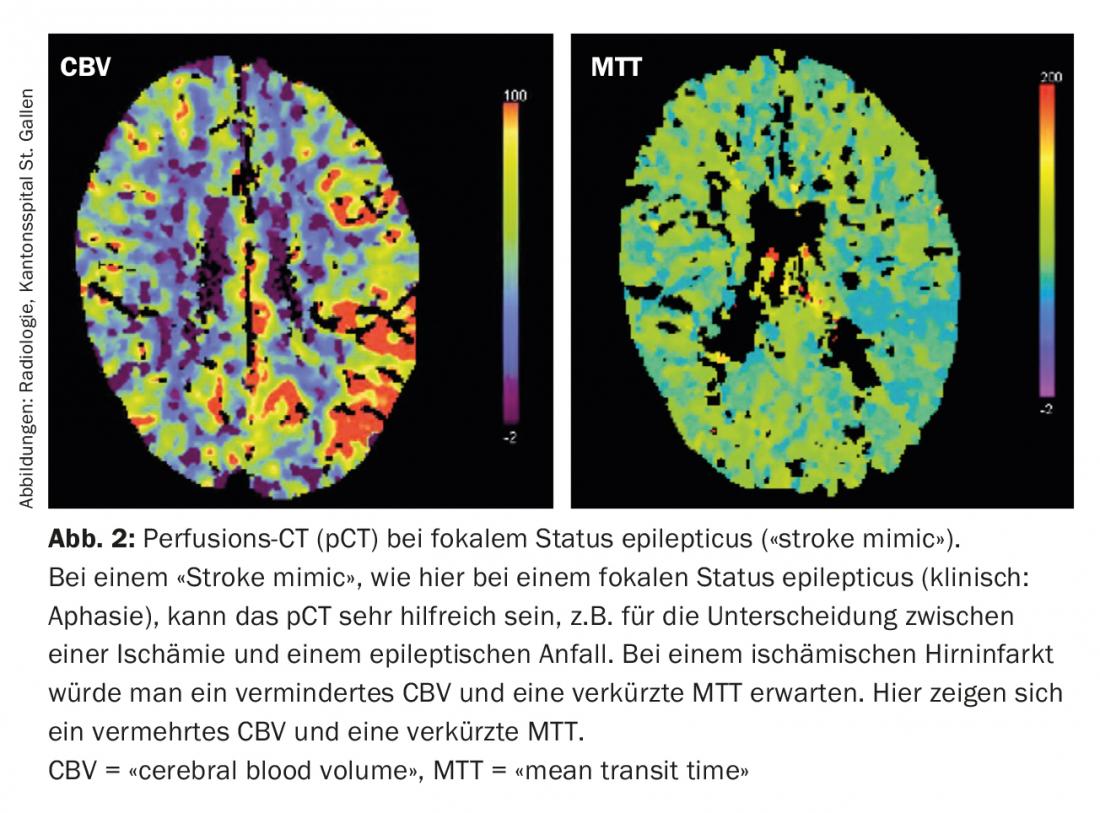
Today, in the acute situation, it is possible to estimate the size of the penumbra and the infarct core with multimodal imaging ( CT, MRI) (Fig. 1) . This information is of therapeutic relevance especially for borderline decisions. In addition, multimodal imaging can provide valuable information in the differentiation of so-called “stroke mimics” (conditions that mimic the image of an ischemic stroke) and thus prevent non-indicated acute therapies (Fig. 2).
Clinical classification of ischemic stroke
Symptoms of ischemic cerebral infarction are divided clinically (Oxford Community Stroke Project Classification) into those of the anterior circulation and those of the posterior circulation, respectively, and into lacunar syndromes. In the anterior circulation, a distinction is made between “Total Anterior Circulation Syndrome” (TACS, 16%) and “Partial Anterior Circulation Syndrome” (PACS, 32%), depending on the extent of the infarct area. Posterior circulation syndrome” (POCS, 21%) is distinguished from this. If a purely motor, sensory, sensorimotor, or ataxic hemisyndrome is present without cortical signs (aphasia, neglect), it is referred to as lacunar syndrome (LACS, 31%) [2].
Infarct etiology
There are several etiologic classifications of ischemic stroke, with the TOAST classification being the best known. It divides stroke into:
- Macroangiopathy: atherosclerotic-related cause of stroke. Usually a symptomatic >50% vascular stenosis of the vessels supplying the brain.
- Cardiac embolic: evidence of at least one relevant cardiac source of embolism (e.g., atrial fibrillation).
- Microangiopathy: Subcortically located cerebral infarcts with a diameter <15 mm.
- Other etiology: for example, vascular dissection, coagulation disorders.
- Unclear etiology: when no cause or multiple competing causes are found [3].
A more recent and differentiated classification is the ASCOD classification, which was proposed in 2009 [4] and presented in a revised version in 2013 [5]. It records and weights all possible causes of stroke. Five phenotypes A (“atheromatosis”/macroangiopathy), S (“small-vessel disease”/microangiopathy), C (“cardiac”/cardiopathy), O (“other cause”/other cause), and D (“dissection”/dissection) are distinguished, each with three degrees of causality. These are: 1. disease present and potential cause, 2. disease present, but causality uncertain, 3. disease present, causality unlikely, 0. disease not present, 9. insufficient clarification to make a classification. A minimum clarification standard is defined for this purpose. Advantages over TOAST classification are: No rigid grouping, no cryptogenic group, differentiated weighting in three levels.
Another recent etiologic concept is cryptogenic embolic ischemic stroke, embolic stroke of undetermined source (ESUS). The operational definition includes diagnostic imaging with exclusion of lacunar infarcts. In addition, ultrasound, CTA, or MRA must be used to exclude hemodynamically relevant stenoses of the brain-supplying vessels in the vascular territory of the current infarct. The minimal cardiac diagnostic test to exclude atrial fibrillation is 24-hour Holter monitoring [6]. Because most strokes meeting ESUS criteria are likely to be embolic in nature and no targeted secondary prevention trials have been conducted for this entity, two large randomized trials of direct oral anticoagulants (dabigatran and rivaroxaban, respectively) versus acetylsalicylic acid are currently underway.
Acute therapy
Prehospitalization phase: Since the successful acute treatment of patients with ischemic cerebral infarction is highly dependent on the latency between symptom onset and treatment initiation (“time is brain”), rapid recognition of and response to stroke symptoms can significantly influence treatment outcome. This applies to the population as well as to the medical staff. The most common symptoms of acute stroke are sudden motor or sensory hemiparesis syndromes, speech disorders, visual field defects or double vision, coordination disorders and also dizziness (Tab. 1). If these symptoms appear, the emergency service should be alerted as soon as possible (telephone 144) and transport to hospital should be arranged, preferably to a specialized center with an acute treatment mandate within a stroke network.

The main reasons of a time delay in the prehospital phase are the lack of knowledge of the population and/or the lack of recognition of stroke symptoms, as well as the insufficient channeling of transport to the nearest hospital with the possibility of acute stroke treatment. In Switzerland, the geographically well-distributed certification of currently 9 stroke centers and 14 stroke units with the required formation of stroke networks has led to a more comprehensive and technically better care of stroke patients. Nevertheless, it still often takes well over an hour before the stroke patient can be given acute therapy.
Immediate on-site measures include: A 30° upper body elevation or a stable lateral position if there is a risk of aspiration. A pulse and blood pressure monitoring, although hypertensive blood pressure values should not be treated until a critical blood pressure limit (systolic >220 mmHg) is exceeded. Determination of capillary blood glucose, keeping the airway clear, and supplemental oxygenation (2-4 L oxygen via nasal cannula) should be sought. In addition, a peripheral intravenous line should be placed. Primary administration of aspirin is not recommended because in the prehospitalization phase it is not possible to distinguish between the different subtypes of stroke, 80-85% of which is ischemic and 15-20% due to hemorrhage.
Hospitalization phase: The primary goal in the treatment of acute stroke is revascularization of the occluded vessel. For this purpose, various therapeutic options with sound scientific evidence of efficacy are available today.
Systemic thrombolysis: After acute diagnosis with neurologic examination and craniocerebral imaging, systemic thrombolysis with intravenous administration of recombinant plasminogen activator (rt-PA) is performed in the first 4.5 hours after symptom onset after review of indications and contraindications. The treatment effect of systemic thrombolysis has been impressively confirmed in several randomized trials and also in a recent meta-analysis, but as already mentioned, it is strongly time-dependent. The “number needed to treat” (NNT) to achieve more of a good functional treatment outcome in a patient increases from 3 in the first 90 minutes to 7 between 0 and 3 hours and to 14 between 3 and 4.5 hours. A good functional treatment outcome means that the patient is able to lead an independent life after the stroke and is not dependent on the help of others. The effect spans all age categories and severity levels [7,8]. If there is an occlusion of a major cerebral vessel, complementary endovascular recanalizing therapeutic procedures have gained acceptance in recent years.
Thrombectomy: Until 2015, convincing evidence of the efficacy of this procedure was lacking. This has changed with the publication of five large studies (MR CLEAN, ESCAPE, REVASCAT, SWIFT PRIME, and EXTEND IA). In all studies, patients with proximal occlusion of an anterior cerebral circulation vessel received either endovascular thrombectomy or systemic thrombolysis within up to 12 hours of symptom onset. Patients with already large established infarcts, posterior stromal infarcts, and already relevant prior disability were excluded. The primary endpoint was functional outcome as measured by the modified Rankin Scale (mRS) at 90 days. Several meta-analyses of these study data have now been published. Accordingly, the odds ratio (OR) of a good functional treatment outcome (mRS 0-2) is 2.42 and the NNT is 5 [9], for an improvement of at least one point on the mRS as low as 2.6. All patient subgroups benefit [10]. It is important to select patients who are not too complex using a radiological cross-sectional imaging technique including angiography. Look for early signs of infarction and provide evidence of proximal vessel occlusion. So-called stent retrievers are the technical standard for treatment. The window of opportunity for treatment is generally up to six hours after symptom onset, or longer in isolated cases. An open question is whether patients should be treated with sedation only or with general anesthesia.
Treatment on a stroke unit: In addition to drug and/or endovascular acute therapy, numerous studies have also shown that treatment on a stroke unit is superior to that on a non-specialized ward in many respects. Mortality in the first year after the event is relatively lower by 18-46% (absolute 3%) and the need for long-term care by 25% [11]. This effect is also demonstrable for all patient groups. A study conducted in Switzerland was able to show that initial treatment in an intensive care unit followed by care by a stroke team without a defined ward was clearly inferior to treatment in a stroke unit that was clearly defined in terms of geography and staffing in terms of treatment outcome after three months [12]. This should be incentive enough to offer the concept nationwide.
Literature:
- Poeck and Hacke 2001, 11th edition, Springer-Verlag Berlin, Heidelberg, New York.
- Bamford J, et al: Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991; 337: 1521-1526.
- Adams HP, et al: Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24; 35-41.
- Amarenco P, et al: A new approach to stroke subtyping: the A-S-C-O (phenotypic) classification of stroke. Cerebrovasc Dis 2009; 27: 502-508.
- Amarenco P, et al: The ASCOD Phenotyping of Ischemic Stroke (Updated ASCO Phenotyping). Cerebrovasc Dis 2013; 36: 1-5.
- Diener HC, et al: Cryptogenic ischemic stroke: time for a paradigm shift in diagnosis and therapy? Act Neurol 2014; 41(01): 35-39.
- Hacke W, et al: Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008; 359(13): 1317-29.
- Emberson J, et al: Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; 384(9958): 1929-35.
- Sardar P, et al: Endovascular therapy for acute ischaemic stroke: a systematic review and meta-analysis of randomized trials. Eur Heart J. 2015; 36(35): 2373-80.
- Goyal M, et al: Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387(10029): 1723-31.
- Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2007.
- Cereda C, et al: Beneficial Effects of a Semi-Intensive Stroke Unit are Beyond the Monitor. Cerebrovasc Dis 2015; 39: 102-109.
InFo NEUROLOGY & PSYCHIATRY 2017; 15(1): 8-11.