Combined radiochemotherapy with classical cytotoxics and specific inhibitors of molecular targets increases the therapeutic breadth and is part of clinical routine in many tumor entities. Classical cytotoxics quantitatively and qualitatively increase DNA damage in combination with ionizing radiation; specific inhibitors of molecular targets increase the efficiency of DNA damage set by radiation. Both tumor cell-specific signal transduction cascades and the tumor milieu (hypoxia, immune system) are interesting targets for modern combined treatment.
Clinical radiotherapy has changed during the last 100 years from an experimental application of X-rays to a complex, technologically highly sophisticated and widely applied therapeutic modality. Probably the most important technological advances that shaped the development of radiotherapy were the introduction of CT and MRI, which allow precise 3D localization of the tumor, and the further development of linear accelerator technology, which nowadays allow precise irradiation of the actual tumor with simultaneous reduction of the irradiation volume and thus sparing of healthy tissue. This development has led to the IMRT (Intensity-Modulated Radiation Therapy) and SBRT (Stereotactic Body Radiation Therapy) technologies used in clinical radiotherapy today. Despite these technical advances, however, unlimited dose escalation without further major normal tissue toxicities will hardly be possible in the future [1].
Combined radiochemotherapy
In parallel with these technological achievements, enormous progress has been made in the field of biological understanding of radiation effects, which are nowadays manifested in the clinic in the field of combined radiochemotherapy. While the combination with classical chemotherapeutic agents aims to increase DNA damage in the cancer cell, modern classes of compounds are designed to increase the efficiency of radiation-induced DNA damage and stress program in the tumor cell and tumor milieu, ultimately leading to tumor death.
We now understand radiation effects in different tumor entities not only at the level of DNA damage, but recognize that the difference in radiation sensitivity in tumor and normal tissue is due to a complex network of intra- and intercellular signal transduction cascades. These findings have led to the development of new strategies to overcome intrinsic as well as acquired radioresistance as part of combined radiochemotherapy with radiosensitizers. These strategies include small-molecule agents that specifically inhibit oncogene-driven signal transduction cascades as well as advanced modulators of immune checkpoints with the goal of increasing therapeutic breadth in the setting of combined radiochemotherapy [2,3].
Currently, more than 50% of all patients with a solid tumor are treated either with radiotherapy alone or as part of a combined therapy with surgery and/or chemotherapy. Currently, 40% of all cancer patients for whom a cure can be achieved also receive radiotherapy as part of a combination therapy.
Although (advanced) head and neck squamous cell carcinoma (HNSCC) affects only a small proportion of all cancer patients, the development of combined radiochemotherapy can be illustrated very well by this tumor entity. At the level of preclinical research, countless combination forms of radiotherapy with small-molecule inhibitors of a wide variety of targets have been tested during the last 20 years. Nevertheless, very few strategies have been successfully implemented in the clinic to date.
Tumor hypoxia
Hypoxia, which is mainly due to the unstructured vascularization of the tumor, is one of the greatest radiation resistance mechanisms in HNSCC. A 2011 level 1a evidence-based meta-analysis clearly demonstrates that a wide variety of hypoxia modification strategies (delivery of hyperbaric oxygen, carbogen breathing in combination with nicotinamide and radiosensitizers, etc.) lead to improved progression-free survival and overall survival in HNSCC [4]. It is therefore all the more surprising that such hypoxia modification strategies have not been part of clinical routine to date – except in Denmark using nimorazole.
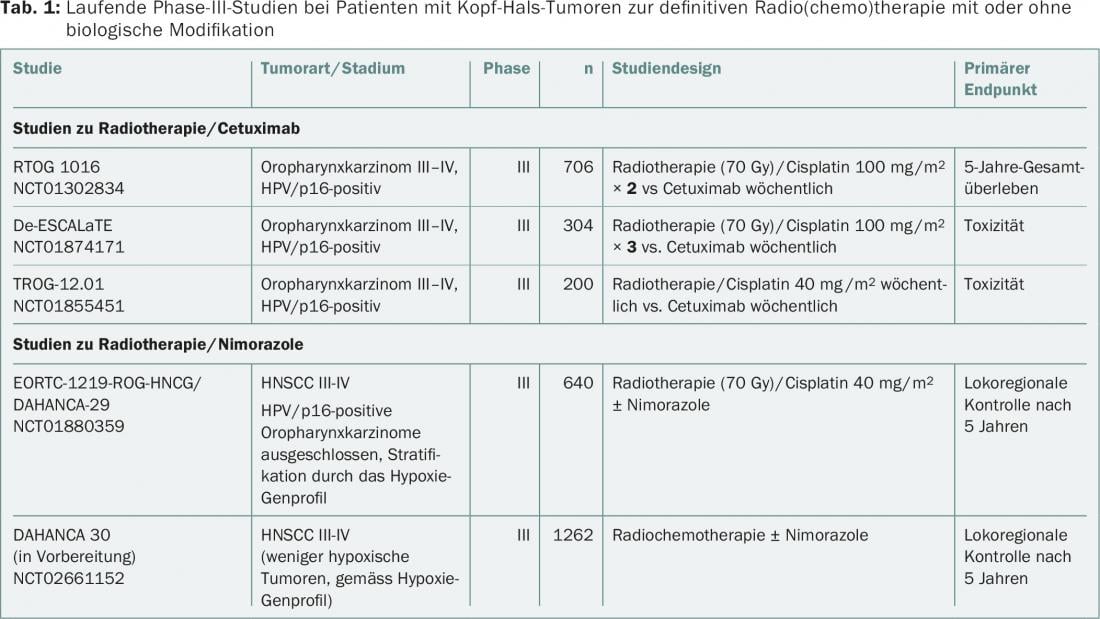
Nimorazole belongs to the class of so-called oxygen mimetics (misonidazole, etanidazole, pimonidazole), which have been clinically tested as prototype hypoxia radiosensitizers for decades. However, most of these nitroimidazole derivatives cause severe neurotoxicity at high doses and are therefore used clinically only as diagnostic tumor hypoxia markers. However, nimorazole appears to be making its way into routine clinical practice, in part because of the experience gained in clinical trials with so-called “hypoxia prodrugs” (tirapazamines, evofosfamide) [5,6]. These studies indicate that characterization of hypoxia status and appropriate stratification should be performed for combined radiochemotherapy with hypoxia radiosensitizers. In general, there are major efforts to predictively determine both the hypoxia status before treatment initiation and the dynamics of tumor hypoxia under radiotherapy using hypoxia PET (e.g., with F18-labeled misonidazole). At the same time, a so-called 15-gene expression signature for hypoxia classification was also identified. This signature characterizes tumors as more or less hypoxic and will complement the conventional direct determination of the partial pressure of oxygen in the tumor with respect to hypoxia characterization. For example, a prospective phase III multicenter EORTC trial (EORTC-1219) is currently evaluating the effect of nimorazole in combination with cisplatin-based radiochemotherapy in locally advanced, HPV/p16-negative HNSCC. It will also be noted whether this 15-gene signature is suitable as a predictive classifier. Another large Phase III trial of nimorazole (DAHANCA30) is in preparation.
EGF receptor as a therapeutic target
Various randomized studies and also clinical routine have shown for more than 15 years that the simultaneous administration of cisplatin in combination with radiotherapy increases both progression-free time and overall survival compared to radiotherapy alone. Mechanistically, this effect is predominantly based on the combined modification of DNA by cisplatin and radiation-generated reactive oxygen radicals, which together lead to complex DNA damage. At the same time, however, this combination also results in severe acute and chronic side effects [7]. This stimulated intensive research into alternative therapies, which led to the combination of radiotherapy with the EGFR-specific monoclonal antibody cetuximab (Erbitux®).
Overexpression of epidermal growth factor receptor (EGFR) is a strong and independent prognostic factor in HNSCC. Cetuximab binds with high affinity to its extracellular domain, thereby blocking the binding of receptor-activating ligands. Cetuximab in combination with radiotherapy was clinically tested in the well-known Bonn trial compared to radiotherapy alone and was characterized by improved progression-free survival and overall survival (49 vs 29 months) as well as greatly reduced normal tissue toxicities [8].
A direct comparison of the two combination regimens (cisplatin/radiotherapy vs cetuximab/radiotherapy) has not been documented to date. In terms of treatment de-escalation, three randomized phase III trials are currently evaluating the value of radiotherapy/cetuximab versus radiotherapy/cisplatin in HPV/p16-positive oropharyngeal carcinoma (RTOG 1016, De-ESCALaTE, TROG-12.01) (Table 1) . Partly due to the overwhelming number of cisplatin/radiotherapy trials – compared to cetuximab/radiotherapy combination trials – cisplatin remains the standard of care in combination with radiotherapy. Cetuximab/radiotherapy is now mainly used in HNSCC patients in high-risk situations, such as intolerance to cisplatin or poor general condition. Interestingly, the triple combination form of cetuximab/cisplatin/radiotherapy did not improve progression-free survival or overall survival in a randomized trial [9].
Although various aspects of the Bonn study have been criticized in the past, it represents a milestone in the development of combined radiochemotherapy. On the preclinical and clinical level, it has been shown for the first time that inhibition of a molecularly strictly defined target structure (EGFR) in the tumor cell leads to radiosensitization. Research into the mechanisms of action of this combination regimen also demonstrated that classic, clinically relevant radiotherapy resistance mechanisms are overcome (tumor repopulation, intrinsic repair capacity, high apoptosis threshold). Interestingly, cetuximab may even control immunomodulatory processes that also contribute to the overall response.
Immunotherapy
Intensive preclinical and clinical research is nowadays focused on radiotherapeutically relevant immunological processes. Radiotherapy has long been characterized as immunosuppressive, yet hematopoietic cells are particularly radiosensitive. Nevertheless, it is shown that irradiation of the tumor also has an immunostimulatory effect.
Ionizing radiation leads to activation of natural killer cells, infiltration of CD8-positive T cells, increased antigen presentation in dendritic cells, and production of immunostimulatory cytokines in the tumor. However, our understanding of these radiation-induced processes is rudimentary [10]. For example, we see entirely different response patterns after fractionated deep dose irradiation and irradiation with high single doses – this in turn makes radiobiological translational research very attractive.
The focus is on the study of radiation-induced immunogenic cell death or the activation of the so-called abscopal effect by radiation therapy. This refers to a systemic effect of irradiation on tumor tissue at non-irradiated sites, mediated by immunological processes. There is great interest in enhancing the clinically rare abscopal effect in the setting of combined radiochemotherapy with immunomodulators. Appropriate combined radiochemotherapy protocols with immune checkpoint inhibitors also exist for HNSCC. However, there are currently many question marks and no conclusive clinical trials have been completed to date in this promising application area.
Several phase I/II trials in patients with HNSCC of radiochemotherapy in combination with immunotherapy are currently registered on the NCI website (https://clinicaltrials.gov) and randomized phase III trials are in preparation.
Conclusion
Modern radiotherapy is a high-tech form of cancer therapy in which each patient receives individualized treatment planning based on clinical parameters (including tumor entity, volume, location). In this context, classical chemotherapeutic agents increase the effectiveness of radiotherapy. In the context of “personalized medicine”, the therapeutic breadth of this individualized form of therapy can be further improved with molecularly defined, specific substances. The aim is to overcome the radioresistance profile based on the individual genetic background of the tumor.
Literature:
- Baumann M, et al: Radiation oncology in the era of precision medicine. Nat Rev Cancer 2016; 16(4): 234-249.
- Begg AC, et al: Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 2011; 11(4): 239-253.
- Morris ZS, et al: Interaction of radiation therapy with molecular targeted agents. J Clin Oncol 2014; 32(26): 2886-2893.
- Overgaard J: Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck – a systematic review and meta-analysis. Radiother Oncol 2011; 100(1): 22-32.
- Ahn GO, et al: Targeting tumors with hypoxia-activated cytotoxins. Front Biosci 2007; 12: 3483-3501.
- Brown JM, et al: Exploiting tumor hypoxia in cancer treatment. Nat Rev Cancer 2004; 4(6): 437-447.
- Pignon JP, et al: Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009; 92(1): 4-14.
- Bonner JA, et al: Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010; 11(1): 21-28.
- Ang KK, et al: Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014; 32(27): 2940-2950.
- Salama AK, et al: Irradiation and immunotherapy: From concept to the clinic. Cancer 2016; 122(11): 1659-1671.
InFo ONCOLOGY & HEMATOLOGY 2016; 4(7-8): 12-15.











