The skin changes that occur in hidradenitis suppurativa (HS), particularly in the intertriginous areas, can be very distressing for those affected. For some years now, research into the complex interrelationships has been stepped up in the hope of reducing the number of cases of HS. Awareness of this condition, which is often associated with comorbidities/concomitant diseases, is crucial in order to be able to provide those affected with adequate treatment.
Hidradenitis suppurativa – also known as acne inversa – is a chronic recurrent disease characterized by painful and recurring lumps, abscesses and fistulas as a result of hair follicle inflammation. “It is a well-known clinical picture, but has only received enough attention in the last two decades,” explained PD Dr. med. Cornelia Erfurt-Berge, senior physician at the Dermatology Clinic of the University Hospital Erlangen [1]. The pathophysiology is now much better understood than it was a few years ago, even if there are still unanswered questions. “What we do know is that there is an excessive inflammatory reaction in the area of the hair follicle and that certain environmental toxins/exogenous factors such as smoking or obesity play a role,” said the speaker. It is assumed that hyperkeratosis ultimately leads to follicular occlusion and that inflammation and bacterial foci form in the surrounding tissue as a result of the closure of the apocrine glands and subsequent rupture of the follicles [1,8].
Proinflammatory signaling pathways are activated
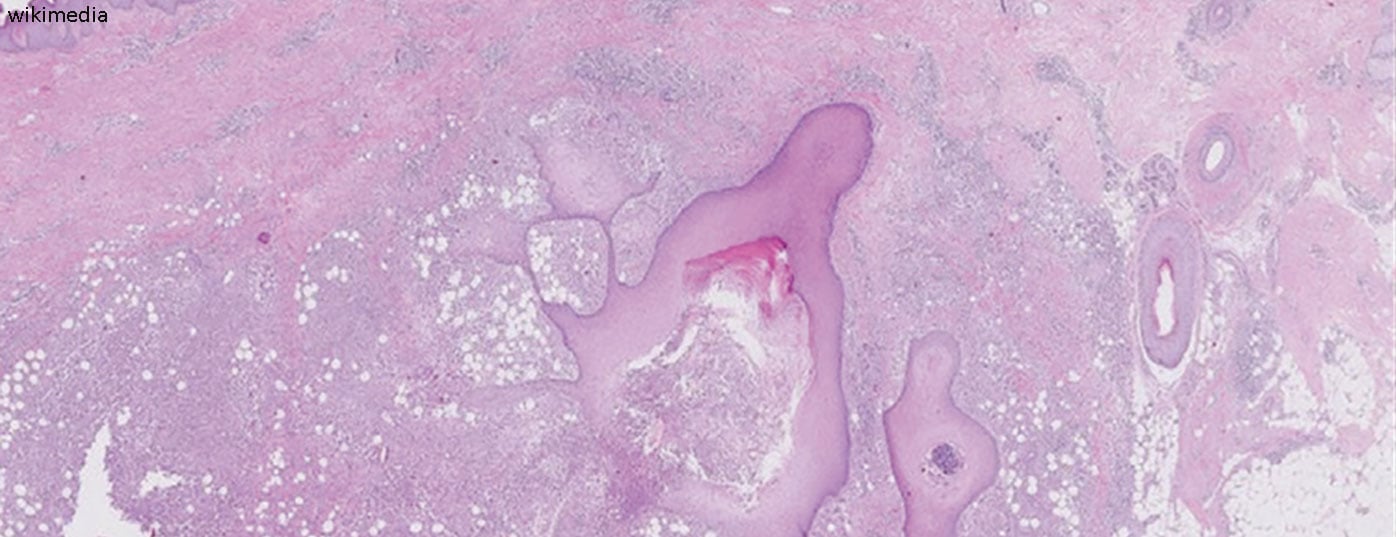
That follicular occlusion initially occurs due to hyperkeratosis can be deduced from histological examinations [2,8]. The occlusion of the apocrine glands and subsequent rupture of the hair follicles induces an inflammatory immune response associated with the recruitment of neutrophils, macrophages, B cells, Th1 and Th17 cells into the skin, resulting in inflammatory nodules or abscess formation [2]. Proinflammatory signaling pathways contribute significantly to the development of HS. In connection with a shift in T cells, there is an increased production of pro-inflammatory cytokines such as TNF-alpha and interleukin (IL)-17, which contribute to the maintenance of inflammation. There is also evidence that toll-like receptors are regulated differently in HS than in healthy skin and that antimicrobial peptides for bacterial defense have an altered expression pattern [1]. “These are complex interrelationships that we are understanding better and better,” Dr. Erfurt-Berge summed up.
Strengthen awareness and avoid diagnostic latency
If HS is not diagnosed in time and treated adequately, secondary complications or changes can develop, such as the formation of fistulous tracts and scars in intertriginous areas (axillary, inguinal, genital). According to the speaker [1], this can lead to considerable movement restrictions. The quality of life of HS patients is often severely restricted. Women and men are affected in roughly equal numbers; in terms of age group, the prevalence is highest among young people, with a mean age of around 23 years [1]. There have been improvements in terms of diagnosis in recent years, but diagnosis latency is still a problem, the speaker emphasized [1]. The diagnosis of HS is mainly made clinically; the time criterion is at least two relapses within six months. The disease is often confused with common abscesses or folliculitis in the early stages.
There are various scores for classifying HS, the most common being the Sartorius score and the Hurley stages [3–5]. With the Sartorius score, the number of inflammatory nodules, abscesses, fistulas and affected areas is evaluated with points. The Hurley classification distinguishes between the following three degrees of severity depending on the clinical manifestation [4,6].
- Stage I: Isolated, single or multiple painful abscesses, no scar strands;
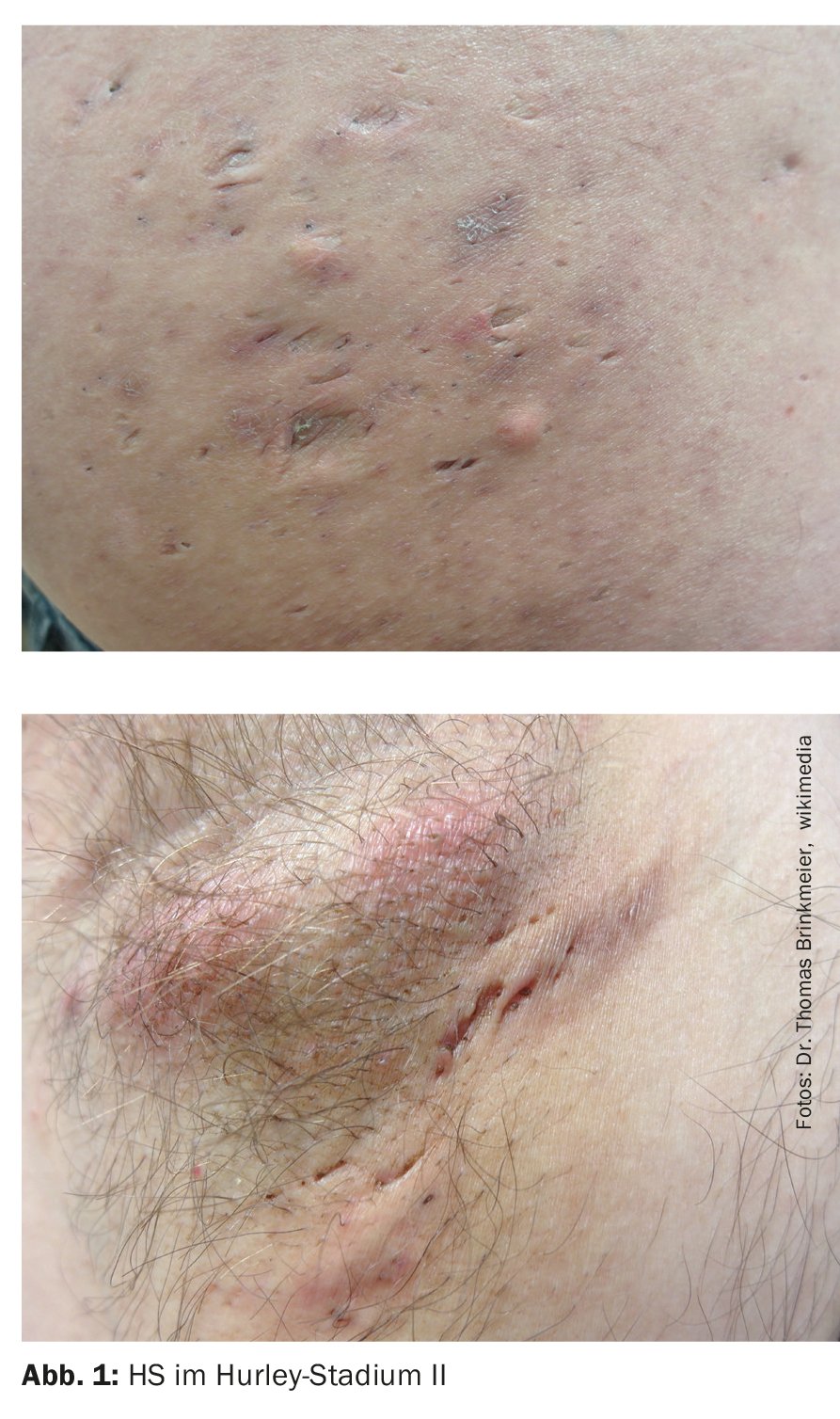
- Stage II: Recurrent painful abscesses with cord formation and scarring, single or multiple, but not extensive (Fig. 1);
- Stage III: Diffuse, squamous, inflammatory, painful infiltrations, or multiple interconnected strands and abscesses. There is a risk of joint contractures as a result of pain-related restriction of movement.
It should also be borne in mind that HS is often associated with comorbidities and concomitant illnesses, the possible presence of which should be investigated. These include joint problems, chronic inflammatory bowel disease (IBD), metabolic syndrome, obesity and diabetes [2,7]. These factors can have a negative effect on HS symptoms. Psychiatric disorders also occur more frequently. Overall, the quality of life with HS is often significantly impaired.
What therapeutic approaches are available today?
If possible, HS patients should be given adequate treatment before the abscess foci coalesce and fistula tracts form. Treatment depends on the severity of the disease. Surgical treatment is still considered the gold standard, especially for severe forms of HS, but systemic drug treatment is becoming increasingly important, according to Dr. Erfurt-Berge. If necessary, surgery and system therapeutics can be combined. Local excision is only an option for Hurley stage I. Pure incisions always lead to recurrences. Another technique is deroofing (electrosurgical, laser). In particularly severe cases (Hurley II or III), a wide “en bloc” resection is required to remove the entire affected area [8]. In addition to clindamycin/rifampicin as conventional systemic therapies, two biologics, adalimumab and secukinumab, are now available for drug treatment and others are currently being investigated in clinical trials [1]. The indication for topically applied clindamycin is limited to Hurley stages I and II. Important accompanying measures in the treatment of HS are lifestyle factors such as weight reduction, smoking cessation and loose clothing [1].
Congress: Wound Congress Nuremberg
Literature:
- “Acne inversa – new insights into a well-known clinical picture”, PD Dr. med. C. Erfurt-Berge, Wundkongress Nuremberg, 23-24.11.2023.
- Vossen A, van der Zee HH, Prens EP: Hidradenitis suppurativa: A systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol 2018; 9: 2965.
- Hunger RE, et al: Swiss Practice Recommendations for the Management of Hidradenitis Suppurativa/Acne inversa. Dermatology 2017; 233: 2-3.
- Revuz J: Hidradenitis suppurativa. JEADV 2009; 23: 985-998.
- Hunger RE, et al: Swiss practice recommendations for the treatment of hidradenitis suppurativa (acne inversa). Compass Dermatol 2019; 7 (1): 8-13.
- Hurley HJ: Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH, Jr. [Eds]. Dermatologic surgery: principles and practice.2nd ed. New York: Marcel Dekker 1996: 623-645.
- Molinelli E, et al: New Insight into the Molecular Pathomechanism and Immunomodulatory Treatments of Hidradenitis Suppurativa. Int J Mol Sci 2023; 24(9): 8428.
- Zouboulis CC, et al. : European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. JEADV 2015; 29(4): 619-644.
DERMATOLOGIE PRAXIS 2024; 34(1): 38-39 (published on 19.2.24, ahead of print)