Immunothrombocytopenia (ITP) is an autoimmune disease that is frequently observed in children and adolescents as well as in people over 60 years of age. It is a diagnosis of exclusion. Especially very low platelet levels are associated with increased morbidity and mortality. Therapy is therefore recommended for platelet levels <30 G/l (or higher if bleeding or risk of bleeding [e.g., in patients taking Aspirin®]) or for rapid platelet decline. Drugs that affect coagulation should be discontinued (e.g., NSAIDs) before specific measures are taken. In newly diagnosed ITP in adults without secondary causes and without severe bleeding, first-line therapy with steroids is the mainstay. Immunoglobulins are used primarily in cases of very low platelet counts and/or significant bleeding. However, they only have a short-term effect. Platelets should be used only in exceptional cases of life-threatening bleeding. In the second line, treatment is individualized. Options range from splenectomy to rituximab, which has no approval in Switzerland for this indication, to the thrombopoietin agonists eltrombopag and romiplostim.
Immunothrombocytopenia (ITP) was also formerly known as idiopathic thrombocytopenic purpura or Werlhof’s disease. However, the term immunothrombocytopenia should be used generically today because it best represents the pathogenesis. ITP is an autoimmune disease that has an incidence of 20-100/million/year, depending on sources [1,2]. The wide dispersion has most likely to do with the fact that ITP is a diagnosis of exclusion and thus, depending on the extent of the workup, not all secondary causes were detected. Generally, a platelet count <100 G/l is required for diagnosis [3].
The disease has two age peaks. One is in children and adolescents, the other in people over 60. ITP is chronic in about one-third of children, whereas in adults a chronic course must be expected in two-thirds. According to the duration of the disease, we speak of newly diagnosed ITP (with a course of less than three months), persistent ITP (with three to twelve months) and chronic ITP (with more than twelve months). Depending on whether a concomitant disease associated with ITP can be found in the workup, it is referred to as secondary ITP or, in the absence of such a disease, as primary ITP.
Clarification of thrombocytopenia
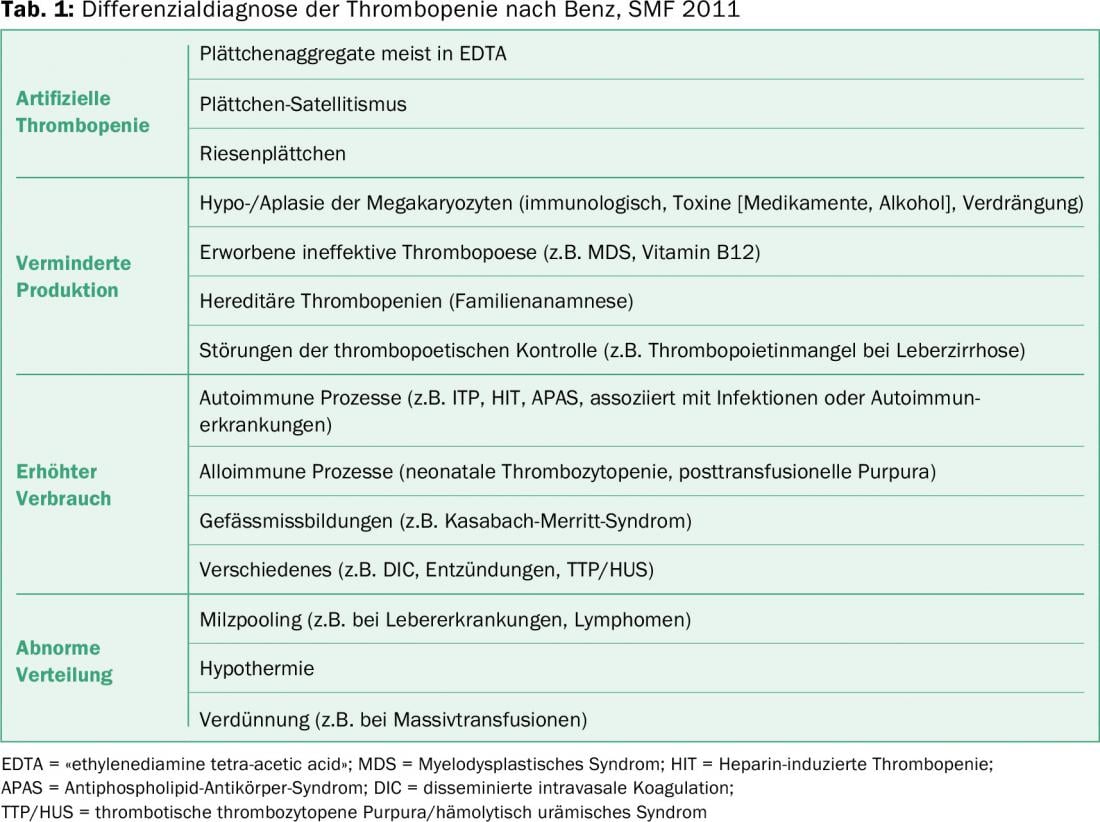
ITP is a diagnosis of exclusion, so other causes of low platelet counts must be ruled out. Table 1 provides an overview of the differential diagnosis of thrombocytopenia. In isolated thrombocytopenia, the first thing to do is to exclude pseudothrombocytopenia. Clinically, these patients do not show bleeding despite severely decreased platelet levels. The diagnosis can be made microscopically and the platelet count from the citrated blood is almost always normal.
If it is determined that thrombocytopenia is not just a laboratory phenomenon, it is usually advisable to perform a more or less standardized workup and laboratory testing (Table 2).

On the one hand, the clarification aims to detect secondary forms and, on the other hand, to detect additional coagulation disorders that further increase the risk of bleeding. Of course, a general clinical examination is recommended. Specifically, however, attention should be paid to bleeding signs, lymphadenopathy, splenomegaly, and liver changes. In childhood, attention should also be paid to changes in the context of congenital syndromal disorders [4]. Often, a positive family history of thrombopenias is also found in congenital disorders. In order to avoid incorrect treatment, great attention must be paid to this point, especially in younger patients.
Likewise, attention should be paid to an accurate medication history [5]. Common drugs such as paracetamol or piperacillin have already been associated with ITP [5]. Heparin exposure must always be actively ruled out, as heparin-induced thrombocytopenia is associated with high morbidity and mortality and can occur even days after the last brief exposure to heparin [6]. Heparin-induced thrombocytopenia is treated differently from ITP as a prothrombogenic condition.
The occurrence of ITP after vaccination can also be observed in clusters and is particularly well known with the MMR vaccination (measles, mumps, rubella) [7]. Since spontaneous recovery virtually always occurs in the course and the rate of ITP is higher in normal infection with these pathogens, this relationship is not an excuse to avoid vaccination. In addition, if there is a history of ITP, vaccinations are not contraindicated. If one is about to receive a second vaccination after e.g. MMR-induced ITP, a vaccination titer determination is recommended to determine the further procedure [8].
Risk and symptoms of ITP
The danger of ITP is bleeding. A relationship between ITP and life expectancy has been demonstrated [9]. Especially very low platelet levels are associated with increased morbidity and mortality [10]. Treatment is therefore recommended for platelet levels <30 G/l (in case of bleeding or increased risk of bleeding, e.g. in case of required medication [e.g. Aspirin®, Marcoumar®], also for higher levels) or in case of rapid platelet decline [8].
In addition to the obvious bleeding, studies in recent years have also shown general fatigue to be a sign of ITP. Whether this fatigue can be explained solely by the defective activity of the immune system or is a consequence of the psychological stress of potential bleeding cannot be said with absolute certainty. Under therapy, fatigue could be positively influenced in any case [11].
Therapy in the first line
Before specific measures are taken, drugs that affect coagulation should be discontinued (e.g., NSAIDs) whenever thrombocytopenia is confirmed. Tranexamic acid may also be considered as an initial nonspecific measure for bleeding. However, the use must always be in proportion to the potential risk of thrombosis, because even with low platelet levels there is a risk of thrombosis.
In newly diagnosed ITP in adults without secondary causes and without severe bleeding, first-line therapy with steroids is the mainstay. In case of possible contraindications to steroids or possible lymphoma, immunoglobulins are recommended as an alternative, as this does not interfere with the assessment of possible lymphoproliferation.
Whenever possible, a serum tube must be collected prior to their administration so that serology results are not altered. However, longer-term control of platelet counts cannot be achieved with immunoglobulins, although the most rapid increase in platelets can usually be achieved with this therapy [12]. Platelets should be used only in exceptional cases of life-threatening bleeding because platelets are also rapidly eliminated by antibodies. When platelets are used, determination of an hourly value is important because it can further support or challenge the diagnosis. The dosages and pre- resp. Disadvantages of the therapies are listed in Table 3 .
Specifically, with prednisone therapy, care should be taken to slowly phase out the therapy [13]. However, because of the longer-term side effects, steroid therapy should be considered a failure if the prednisone dose cannot be reduced below 10 mg/d without platelets returning to levels requiring treatment. To reduce these side effects, therapy with dexamethasone 40 mg/d was introduced for four days every 28 days [14]. This therapy appears to have a slightly better longer-term remission rate (up to 50%).

Therapy in the second line
The choice of further therapy is to be made on an individual basis. Splenectomy continues to have a very good long-term response and can nowadays be performed with laparoscopic methods with great safety [15]. In addition to early perioperative morbidity and mortality, longer-term risks of postsplenectomy sepsis (OPSI) are the most feared. Although this can be greatly reduced by mandatory pneumococcal, meningococcal, and hemophilus vaccinations, it cannot be completely prevented. Parasitic diseases are also more dangerous after splenectomy [16]. The risk of pulmonary hypertension, a complication that has been observed after splenectomy in patients with erythrocyte membranopathies, has not been systematically studied in the setting of ITP [17,18]. Because of the above-mentioned reasons, splenectomy is usually performed only in the third or fourth line of therapy despite very good long-term success (two-thirds therapy-free patients).
Alternatively, rituximab, which has no approval in Switzerland for this indication, or the thrombopoietin agonists eltrombopag and romiplostim are used. Rituximab shows a good safety profile. However, hepatitis B must be excluded prior to therapy, and use in patients with variable humoral immunodeficiency syndrome (CVID) must be assessed on an individual basis. Although a recent randomized trial did not show a higher response rate in the second line of therapy versus placebo [19]. However, the patients who responded were able to maintain the response longer. The longer-term response rate is limited but can be improved with concomitant administration of dexamethasone and, at most, ciclosporin [20]. The great advantage is the limited time of administration. In contrast, TPO agonists show a very good response rate, but must be given indefinitely, except in a few isolated cases, to maintain response [21,22]. Likewise, a latency of action of about one week should be noted, so that the therapy is not an emergency option.
There is no direct comparison of the two treatments, so that the mode of administration in particular is an important point for the choice of therapy. Romiplostim is administered subcutaneously once a week. There are no interactions with food or other medications and gastrointestinal tolerance is not a problem. Eltrombopag can be easily administered as a tablet, which may be an argument, for example, in patients with busy travel schedules. Conversely, certain eating restrictions (polyvalent cations) and interactions must be considered.
Specifically, the impact on constitutive symptoms and quality of life was investigated for the new drugs. Both parameters could be positively influenced [23]. Whether reduced performance in the absence of an indication for drug therapy should in itself be considered an indication is an open question. In any case, the item is not listed in the list of indications.
Newly, increased desialylation was found in GPIbIX antibodies, leading to platelet degradation via the Ashwell-Morell receptor in the liver, explaining the reduced effect of various therapies [24,25]. Whether the use of neuraminidase inhibitors could have a positive impact here is an open question [26].
Literature:
- Cines DB, Blanchette VS: Immune thrombocytopenic purpura. N Engl J Med 2002; 346(13): 995-1008.
- Moulis G, et al: Epidemiology of incident immune thrombocytopenia: a nationwide population-based study in France. Blood 2014; 124(22): 3308-3315.
- Rodeghiero F, et al: Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 2009; 113(11): 2386-2393.
- Lambert MP: What to do when you suspect an inherited platelet disorder. Hematology Am Soc Hematol Educ Program 2011; 2011: 377-383.
- Reese JA, et al: Identifying drugs that cause acute thrombocytopenia: an analysis using 3 distinct methods. Blood 2010; 116(12): 2127-2133.
- Refaai MA, et al: Delayed-onset heparin-induced thrombocytopenia, venous thromboembolism, and cerebral venous thrombosis: a consequence of heparin “flushes”. Thromb Haemost 2007; 98(5): 1139-1140.
- Rajantie J, et al: Vaccination associated thrombocytopenic purpura in children. Vaccine 2007; 25(10): 1838-1840.
- Neunert C, et al: The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011; 117(16): 4190-4207.
- Cohen YC, et al: The bleeding risk and natural history of idiopathic thrombocytopenic purpura in patients with persistently low platelet counts. Arch Intern Med 2000; 160(11): 1630-1638.
- Portielje JE, et al: Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood 2001; 97(9): 2549-2554.
- George JN, et al: Improved quality of life for romiplostim-treated patients with chronic immune thrombocytopenic purpura: results from two randomized, placebo-controlled trials. Br J Haematol 2009; 144(3): 409-415.
- Imbach P: Immune thrombocytopenic purpura and intravenous immunoglobulin. Cancer 1991; 68(6 Suppl): 1422-1425.
- Neunert C, et al: The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011; 117(16): 4190-4207.
- Cheng Y, et al: Initial treatment of immune thrombocytopenic purpura with high-dose dexamethasone. N Engl J Med 2003; 349(9): 831-836.
- Kojouri K, et al: Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood 2004; 104(9): 2623-2634.
- Demar M, et al: Plasmodium falciparum malaria in splenectomized patients: two case reports in French Guiana and a literature review. Am J Trop Med Hyg 2004; 71(3): 290-293.
- Das A, et al: Risk factors for thromboembolism and pulmonary artery hypertension following splenectomy in children with hereditary spherocytosis. Pediatr Blood Cancer 2014; 61(1): 29-33.
- Schwartz J, et al: Long term follow-up after splenectomy performed for immune thrombocytopenic purpura (ITP). Am J Hematol 2003; 72(2): 94-98.
- Ghanima W, et al: Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015; 385(9978): 1653-1661.
- Choi PY, et al: A novel triple therapy for ITP using high-dose dexamethasone, low-dose rituximab, and cyclosporine (TT4). Blood 2015; 126(4): 500-503.
- Cheng G, et al: Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet 2011; 377(9763): 393-402.
- Bussel JB, et al: AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med 2006; 355(16): 1672-1681.
- Kuter DJ, et al: Health-related quality of life in nonsplenectomized immune thrombocytopenia patients receiving romiplostim or medical standard of care. Am J Hematol 2012; 87(5): 558-561.
- Li J, et al: Severe platelet desialylation in a patient with glycoprotein Ib/IX antibody-mediated immune thrombocytopenia and fatal pulmonary hemorrhage. Haematologica 2014; 99(4): e61-63.
- Li J, et al: Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat Commun 2015; 6: 7737.
- Shao L, et al: Successful treatment with oseltamivir phosphate in a patient with chronic immune thrombocytopenia positive for anti-GPIb/IX autoantibody. Platelets 2015; 26(5): 495-497.
InFo ONCOLOGY & HEMATOLOGY 2016; 4(2): 6-9.