What do so-called targeted therapies actually aim at? And what progress has been made in the various hematologic oncologic neoplasms in this regard? At the 21st Symposium Medicine in the Manege at the Circus Knie, Prof. Dr. med. Markus G. Manz from the University Hospital Zurich gave answers to the most burning questions.
According to Prof. Markus G. Manz, MD, Clinic for Hematology, University Hospital Zurich, cancers in the field of hemato-oncology account for about 10% of neoplasms in Switzerland. These are mainly so-called “old-age diseases”, i.e. conditions that become significantly more frequent with increasing age.
“Even if we assume an intermediate demographic scenario rather than the most extreme, a doubling of the population over 65 years of age will take place by 2030. Thus, in a very simplified way, approximately a doubling of hemato-oncological neoplasms can also be expected, which makes the need for appropriate therapies all the greater,” Prof. Manz explained.
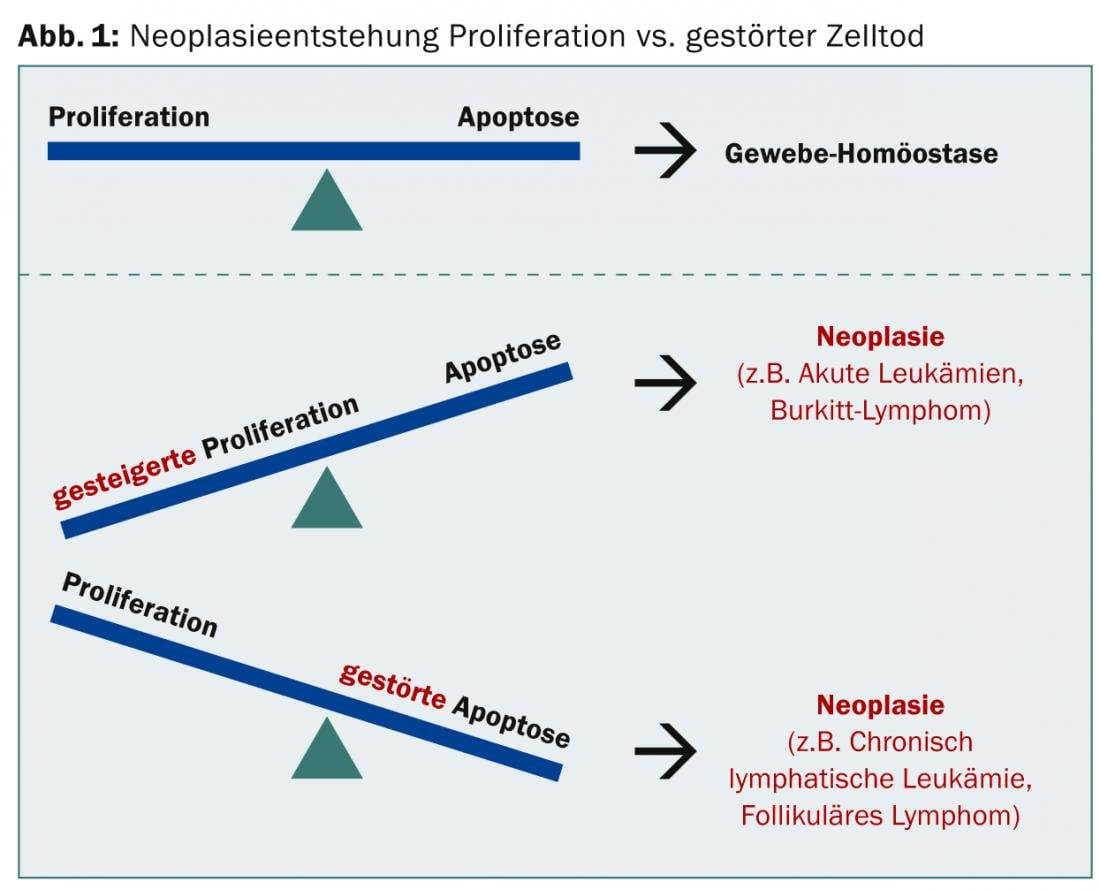
In the case of neoplasia, either increased proliferation or impaired apoptosis may predominate; in either case, tissue equilibrium is no longer present (Fig. 1).
The ideal of targeted therapy, on the other hand, is to achieve the highest possible tumor selectivity; it should attack only the tumor and not the entire patient or healthy tissue. “The so-called non-targeted, classical chemotherapy is usually cell cycle dependent, i.e. it follows the principle: much division – high effect,” he said. Targeted therapy, on the other hand, targets so-called “tumor drivers” that are essential for the tumor and thus destroys the tumor efficiently and, at least theoretically, with as few side effects as possible.
The two most common targets are enzymes/kinases (intracellular) and cell surface antigens. The former are inhibited by so-called “small molecules”, while the latter are attacked by antibodies with or without attached “ammunition”.
Kinases transfer a phosphate residue and can therefore activate other enzymes. They are essential for signal transduction in the cell. In tumor tissue, the kinase is always active, leading to massive cell division. The inhibitor blocks the kinase, thereby placing cell division in an inactive state. “The prime example of a kinase inhibitor is imatinib. Rituximab, on the other hand, is the best-known representative of the monoclonal antibodies and led to a veritable revolution in all lymphomas with CD20 expression, which includes virtually all B-cell lymphomas,” says Prof. Manz.
What improvements can be made?
Using chronic myeloid leukemia (CML) as an example, Prof. Manz showed that since tyrosine kinase inhibitors were first introduced around 2001, treatment efficacy has increased sharply and long-term survival has improved significantly as a result. “About 5% of CML patients on imatinib are so-called super-responders: here, for the first time, the question of a possible cure arises [1,2]”, says Prof. Manz. “The result of the success is an increased expectation in the field of CML for the near future: 100% long-term survival with 0% reduction in quality of life.”
As a second example, he mentioned the development in the field of primary myelofibrosis (PMF): in about 50% of PMF cases, the so-called JAK-2 mutation occurs (Janus kinase 2). Less than ten years after the discovery and identification of this mutation, the first JAK inhibitor was then approved. “Data show under ruxolitinib (Jakavi®), compared to best available therapy, an improvement in symptom control and quality of life [3] and better overall survival compared to placebo [4]. But: JAK-1&2 inhibitors are also active in non-mutated JAK-2. Is then the selective target in the tumor not relevant at all?”, Prof. Manz asked the question.
The last topic was chronic lymphocytic leukemia (CLL). Here, the addition of an anti-CD20 antibody to chemotherapy has been shown to be more efficient than chemotherapy alone. In addition, optimized anti-CD20 antibodies such as GA101 (obinutuzumab) work better than first-generation antibodies [5].
“Currently, there are also studies investigating the efficacy of kinase inhibition in CLL [6]. A broad application of this mode of action in B-non-Hodgkin lymphoma is very likely in the near future,” Prof. Manz concludes.
Source: 21st Symposium Medicine in the Manege, June 5, 2014, Zurich
Literature:
- Mahon FX, et al: Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol 2010 Nov; 11(11): 1029-1035.
- Ross DM, et al: Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood 2013 Jul 25; 122(4): 515-522.
- Harrison C, et al: JAK Inhibition with Ruxolitinib versus Best Available Therapy for Myelofibrosis. N Engl J Med 2012; 366: 787-798.
- Verstovsek S, et al: Long-Term Outcomes Of Ruxolitinib Therapy In Patients With Myelofibrosis: 3-Year Update From COMFORT-I. Blood 2013; 122(21): 396.
- Goede V, et al: Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014; 370: 1101-1111.
- Byrd JC, et al: Ibrutinib versus ofatumumab in Previously Treated Chronic Lymphoid Leukemia. NEJM May 31 2014. DOI: 10.1056/NEJMoa1400376.
InFo ONCOLOGY & HEMATOLOGY 2014; 2(6): 33-34.