The fact that IL17 inhibitors are characterized by a rapid onset of action has now been empirically proven several times and is also reflected in a recent head-to-head study. Looking ahead, there are exciting developments in the pipeline, including the new antibody Bimekizumab, which targets the IL-17A and IL-17F subunits and has shown promising results in recent studies.
The first biologics approved for the treatment of psoriasis were the TNF-alpha inhibitors adalimumab, etanercept and infliximab. While this already represented a significant improvement in treatment options, the majority of patients did not achieve complete freedom from manifestations. In the meantime, however, there has been enormous progress in this regard.
anti-IL17: rapid onset of action as a trademark
The IL12/23 inhibitor ustekinumab was followed by the two IL17A inhibitors secukinumab and ixekinumab, and the IL17 receptor antagonist brodalumab (currently not approved in Switzerland) [1]. IL17 blockade proved to be very effective and allowed optimization of response rates. The latest generation of highly effective biologics also includes the IL23 inhibitors guselkumab, tildrakizumab and risankizumab. There are some differences between the substance classes. “anti-IL17 therapy works fast, very fast,” emphasizes Prof. Diamant Thaci, MD, Institute and Center of Excellence, University of Lübeck [2]. This was already evident from the CLEAR trial published in 2015, in which the IL17A inhibitor secukinumab showed a PASI75 response early, at week 4 after baseline [3]. After 16 weeks of therapy, secukinumab proved superior to the IL12/23 inhibitor ustekinumab in terms of PASI90 response (79% vs. 57.6%; p<0.0001) and achieved greater and more sustained improvement in skin condition by week 52.
The finding of a rapid onset of action has since been replicated several times, and data from a head-to-head study published in 2020 confirm this again. The IXORA trial is a randomized, double-blind study that included patients with moderate-to-severe plaque psoriasis (PASI ≥12, sPGA ≥3, and ≥10% affected body surface area) [4]. The primary end point was PASI100 at 12 weeks, and secondary end points included other PASI and sPGA scores. Of the 1027 patients included in the study, 520 received ixekizumab and 507 received guselkumab. 41% of patients on ixekizumab achieved PASI100 at 12 weeks, compared with only 25% on guselkumab. Overall, ixekizumab proved superior to guselkumab. The onset of action was faster and more patients achieved appearance-free skin, and the safety profiles were comparable. These results underline the rapid response, but do not make any statement about the long-term effect; this is the subject of other studies.

Place of Secukinumab in the Treatment of PsA
In the current EULAR treatment recommendations, TNF-alpha inhibitors and IL17 inhibitors are mentioned as equivalent first-line biologics after failure of conventional DMARDS [5]. This assessment is based on the results of the EXCEED comparative study of first-line therapy in psoriatic arthritis. The double-blind phase IIIb multicenter study included 853 adult patients with active psoriatic arthritis. 426 patients received 300 mg secukinumab subcutaneously at baseline, weeks 1, 2, 3, 4, and then every 4 weeks until week 48. Adalimumab was administered subcutaneously every 2 weeks to 427 patients. With secukinumab, 67% and with adalimumab, 62% of study participants achieved ACR20 at 52 weeks [6]. The safety profile of secukinumab and adalimumab was largely consistent with previously known safety signals. Individual patient characteristics (e.g., comorbidities) are suggested as decision criteria for whether to use secukinumab or adalimumab after failure of a csDMARDS.
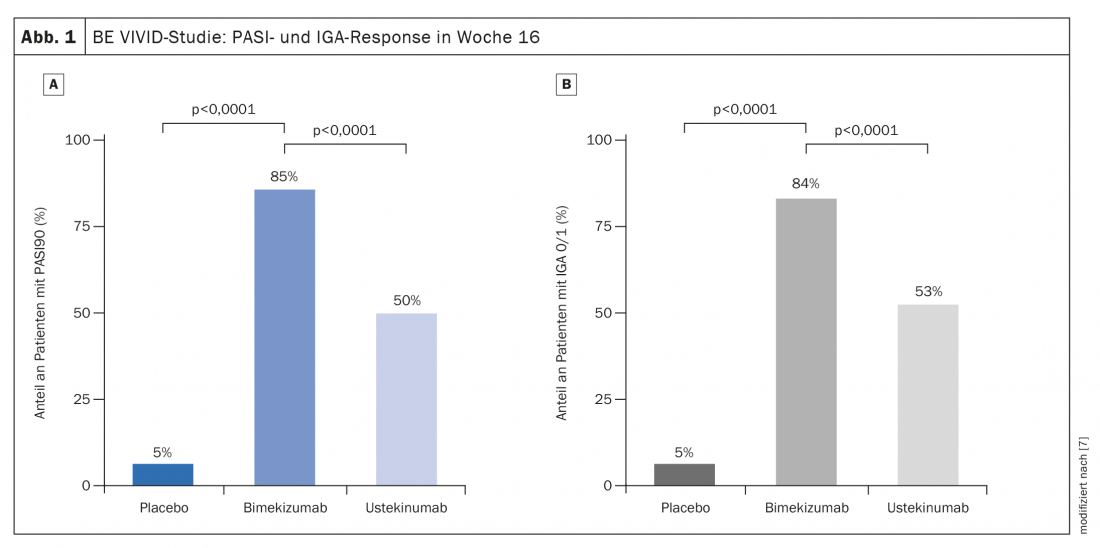
Outlook: promising results of IL-17A/F blockade.
In a comparative study published in 2021, the anti-IL17A/F inhibitor bimekizumab briled with a very rapid onset of action and low loss of effect during the course of the study. Bimekizumab achieved in terms of PASI75 within 4 weeks what ustekinumab achieved only after 4 months, summarized Prof. Thaci. The randomized, double-blind, active, placebo-controlled, multicenter comparative BE VIVID study included 567 adults [7]. 321 study participants received bimekizumab 320 mg every 4 weeks and 163 subjects were treated with ustekinumab 45 mg or 90 mg (weight-adjusted) every 12 weeks; 83 patients received placebo every 4 weeks. At week 16 after baseline, 85% of patients in the bimekizumab arm achieved PASI90, compared with 50% in the ustekinumab arm (risk difference 35 [95%-KI 27–43]; p<0.0001) (Fig. 1A). In the placebo group, this proportion was 5% (risk difference 80 [95%-KI 74–86]; p<0.0001). Approximately 84% of patients on bimekizumab had an IGA score of 0 or 1 compared to 53% on ustekinumab (risk difference 30 [95%-KI 22–39]; p<0.0001) and 5% on placebo (risk difference 79 [95%-KI 73-85] p<0.0001) (Fig. 1B) . Bimekizumab also achieved a faster response. At week 4, after a single dose, a PASI75 score was observed in 77% of patients in the bimekizumab group, compared with only 15% under ustekinumab (OR 18.2; 95% CI 11-30.1, p<0.0001). It was also remarkable that the PASI90 values were not only significantly better after 4 months, but that there was hardly any loss of efficacy under bimekizumab in the further course of the study. This points to a promising potential for anti-IL17 long-term therapy, the speaker said.
Congress: DDG Conference 2021
Literature:
- Sawyer LM, et al: Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: A systematic review and network meta-analysis of PASI response. PLoS One 2019; 14(8): 1-31.
- Thaci D: Biologics in changing times – therapeutic approaches yesterday, today and tomorrow. Prof. Dr. med. Diamant Thaci, S09: Track Inflammations: Atopic Dermatitis and Psoriasis as Systemic Diseases, DDG Conference 2021, 17.04.2021.
- Thaci D, et al: Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. JAAD 2015; 73(3): 400-409.
- Blauvelt A, et al: IXORA-R Study Group. A head-to-head comparison of ixekizumab vs guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial. Br J Dermatol 2020; 182(6): 1348-1358.
- Gossec L, et al: EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020; 79(6): 700-712.
- McInnes IB, et al: Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. The Lancet 2020; 395(10235): 1496-1505.
- Reich K, et al: Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet 2021; 397(10273): 487-498.
- NCT03440736, https://clinicaltrials.gov/ct2/show/NCT03440736
DERMATOLOGIE PRAXIS 2021; 31(3): 20-22 (published 6/1/21, ahead of print).