Upsets in first-line therapy of non-small cell EGFR-mutated lung cancer are imminent. With the FLAURA trial, osimertinib is confidently presenting itself for the first time as a treatment option for therapy-naïve patients.
Hardly an oncology conference goes by without weighty results from the field of non-small cell lung cancer (NSCLC). Not surprisingly, studies on NSCLC formed a core part of the presentations at this year’s ESMO congress. This time it involved first-line treatment of advanced patients with EGFR mutations.
Short review
In 2016, as usual, InFo ONCOLOGY & HEMATOLOGY reported from the European Lung Cancer Conference in Geneva. At that time, two late-breaking abstracts had demonstrated the efficacy of osimertinib, a potent third-generation selective tyrosine kinase inhibitor (anti-EGFR), in the above population. Partially pooled study results from Phase I and II were involved. The studies in question were named AURA P1, AURA extension and AURA 2. they unanimously concluded that osimertinib at the dose of 80 mg/d in patients with advanced NSCLC and the EGFR T790M mutation who had progressed on prior EGFR TKI therapy, provided a high response rate over a relatively long duration with encouraging progression-free survival and a manageable side effect profile. Why is this important? Now, when a driver mutation of EGFR is detected, which applies to about 15% of all NSCLC cases in the Western world and just over a third in the Asian world, specific targeted tyrosine kinase inhibitors (TKIs) have been available for some time and have achieved good results. However, resistance development and disease progression occurs under these TKIs – in a majority of all resistance, the gatekeeper mutation T790M is the cause. Osimertinib as an inhibitor of EGFR with sensitizing mutations (EGFRm) and the TKI resistance mutation T790M fills this gap and was therefore gratefully included in the therapeutic regimen. Accordingly, osimertinib has also been approved in the second-line setting in Switzerland since the middle of last year.
First line as target
Even then, however, it became apparent that osimertinib would also make inroads into the first-line setting. Another presentation at ELCC 2016 with positive data from 60 therapy-naive patients suggested as much. Osimertinib delays resistance. The tumor must obviously seek new resistance mechanisms in addition to the EGFR-T790M mutation. So what happens if you block this central “escape route” for the tumor right from the start?
Final clarity should be provided by a phase III trial with over 500 patients that compared osimertinib with erlotinib and gefitinib (“standard of care”). The results were expected in about one and a half years – at ESMO 2017 the time had come. This much in advance: The presentation was convincing. It is likely that osimertinib will soon be available in the first-line setting.
FLAURA – the success story continues
The phase III trial in question bears the resounding name FLAURA. Participants included 556 adults from Asia, Europe and North America with advanced NSCLC with activating mutations of EGFR-TK, including deletion in exon 19 and the point mutation L858R in exon 21. They were randomized to receive the “standard of care” in the first-line setting, which is gefitinib 250 mg/d or erlotinib 150 mg/d was defined, or osimertinib 80 mg/d. Crossover to osimertinib was possible at progression and T790M resistance. Baseline patient characteristics such as mutation status, sex, CNS metastases, and ethnicity (Asian, non-Asian) were evenly distributed across arms.
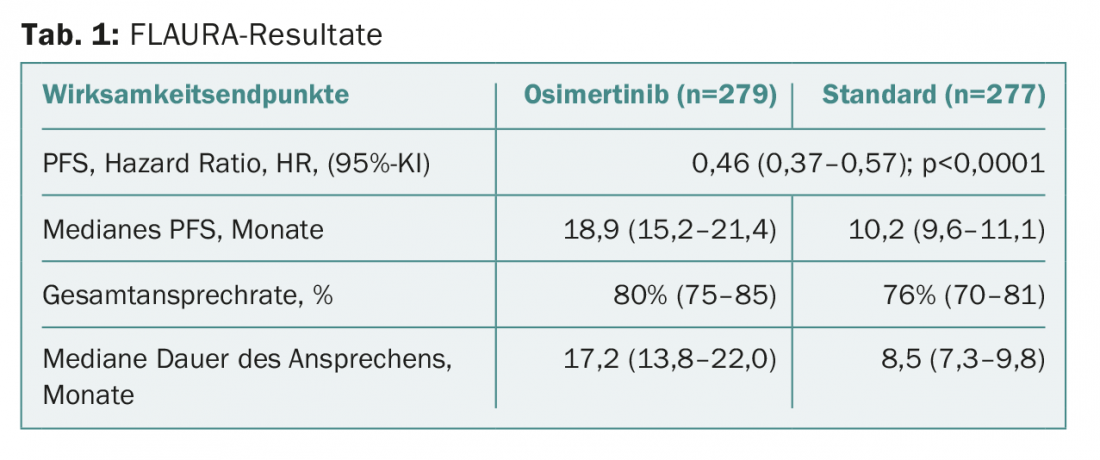
The primary endpoint, progression-free survival, was extended to 18.9 months with the test substance compared to 10.2 months with standard therapy. This corresponds to a clinically and statistically significant risk reduction by more than half. The values are shown in table 1 . Benefit was seen in all predefined subgroups (including patients with/without brain metastases at baseline). A corresponding final analysis on overall survival is currently pending. The interim analysis had not yet revealed any statistically significant values. At this early stage, the data is still “immature”. It is therefore not yet possible to make a conclusive statement in this regard. The duration of response was doubled with osimertinib.
As already known from previous studies, the most frequent adverse events with osimertinib included diarrhea (58%, in 2% at least of grade 3) or dry skin (32%). Overall, adverse events of any cause were equally frequent in the two arms. Severe cases occurred more frequently under standard therapy (which, incidentally, was also true for the corresponding therapy discontinuation rate: 13% vs. 18%). Overall, osimertinib therefore performs better in terms of safety.
Does everyone agree?
The authors inferred a superior risk-benefit profile for osimertinib in the first-line setting from the data. The safety profile was clearly better despite an overall longer treatment duration with the test substance (16.2 months). That the benefit in PFS with/without brain metastases was almost equal (HR 0.47 and 0.46, respectively) suggests that osimertinib is active in the brain as well as systemically. This is particularly important for EGFR-mutated tumors, as they often present with brain metastases. The brain activity results are supported by the finding that 6% in the osimertinib group but 15% in the standard group developed CNS progression. It was also noteworthy that the PFS curves diverged significantly early on and continued to differ clearly throughout the course. The trend in overall survival also appears promising. So it looks like a paradigm shift.

Was the response among experts and visitors to the conference just as positive? Majority do. First-line relevance was clearly conceded to FLAURA. Comparisons were made to the situation in tumors with ALK mutation and the corresponding study on alectinib – recently published in NEJM [1]. There, too, the question was whether first-line use was superior to “sequencing” the agents. Specifically, this means that a longer PFS is achieved with the original second-line agent in the first line than would be possible with sequencing. However, while the situation for ALK is very clear, the situation for FLAURA is somewhat more complex. This is because only about half of the FLAURA study population would have needed and benefited from second-line osimertinib after first-line therapy with established agents (as they would have developed resistance via T790M). Given the fact that the study thus also includes quite a few patients who would never have developed such resistance but would have developed other resistance, one would not expect a median PFS of 19 months. Such long periods are usually only achieved with patients suitable for osimertinib sequencing (this is not statistically true for about 50% of the FLAURA population). For all others, shorter periods can be assumed, as they can often only switch to chemotherapy after first-line therapy. Given this, 19 months of progression-free survival is impressive and indeed argues for first-line use in this population. In particular, because it is never certain whether it will be possible to switch to osimertinib or other specific second-line therapy at all – as it remains unclear at the start of initial therapy whether patients will develop resistance such as T790M and thus benefit from the specific agents – the 19 months with osimertinib that can be achieved up front may facilitate the decision to use it in the first line. Freely according to the principle: “the winner takes it all”.
However, it might not be that simple after all. In this context, the further development of the OS in FLAURA is also important. The last word has not yet been spoken in EGFR-mutated tumors.
Source: European Society for Medical Oncology (ESMO) 2017 Congress, September 8-12, 2017, Madrid.
Literature:
- Peters S, et al: Alectinib versus crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017; 377: 829-838.
- Eggermont AM, et al: Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. NEJM 2016; 375: 1845-1855.
- Weber J, et al: Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. NEJM 2017 September 10. DOI: 10.1056/NEJMoa1709030 [Epub ahead of Print].
- Long GV, et al: Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. NEJM 2017 September 10. DOI: 10.1056/NEJMoa1708539 [Epub ahead of print].
InFo ONCOLOGY 2017; 5(5): 35-37.











