The guidelines of the European and American urological societies for localized prostate cancer show that radical prostatectomy, “low dose rate” (LDR) brachytherapy, and external beam radiotherapy have comparable efficacy in terms of PSA recurrence-free survival over the long-term. The well-informed patient will therefore choose his therapy mainly on the basis of the side effects of the method. However, data regarding urinary incontinence, erectile function, bladder voiding dysfunction, and rectal toxicity are sparse and comparisons are difficult. In Switzerland, all brachytherapies have been prospectively recorded in a national registry since January 1, 2005. The ongoing evaluation paints a consistently positive picture for LDR brachytherapy.
Three established procedures are available for the treatment of clinically localized prostate cancer (T1-2 N0 M0): radical prostatectomy, “low dose rate” (LDR) brachytherapy, and percutaneous radiotherapy [1,2]. Other therapeutic options such as cryotherapy, high-intensity focused ultrasound (HIFU), interstitial tumor ablation with radiofrequency (RITA), and “high dose rate” (HDR) brachytherapy (afterloading with iridium-192) are still considered experimental because, on the one hand, only few case-control studies exist and, on the other hand, no long-term follow-up is available.
Advising a patient regarding therapy depends on several factors: Efficacy of the type of therapy (long-term oncologic outcome), side effects, preference of the patient and the treating physician, and other factors such as cost of treatment, length of hospitalization, etc. A prospective randomized study comparing the three types of therapy does not exist and will not exist in the future. The patient is confronted with a wealth of non-validated information (lay press, Internet), and the treating physician is not infrequently inclined to cite those scientific studies which show his preferred type of therapy in a good light.
Based on encouraging reports from the USA, the Clinics of Urology and Radio-Oncology at the Cantonal Hospital St. Gallen, in close cooperation, were the first in Switzerland to start LDR brachytherapy in March 2001 [3]. Eleven more centers were subsequently added.
LDR brachytherapy technique
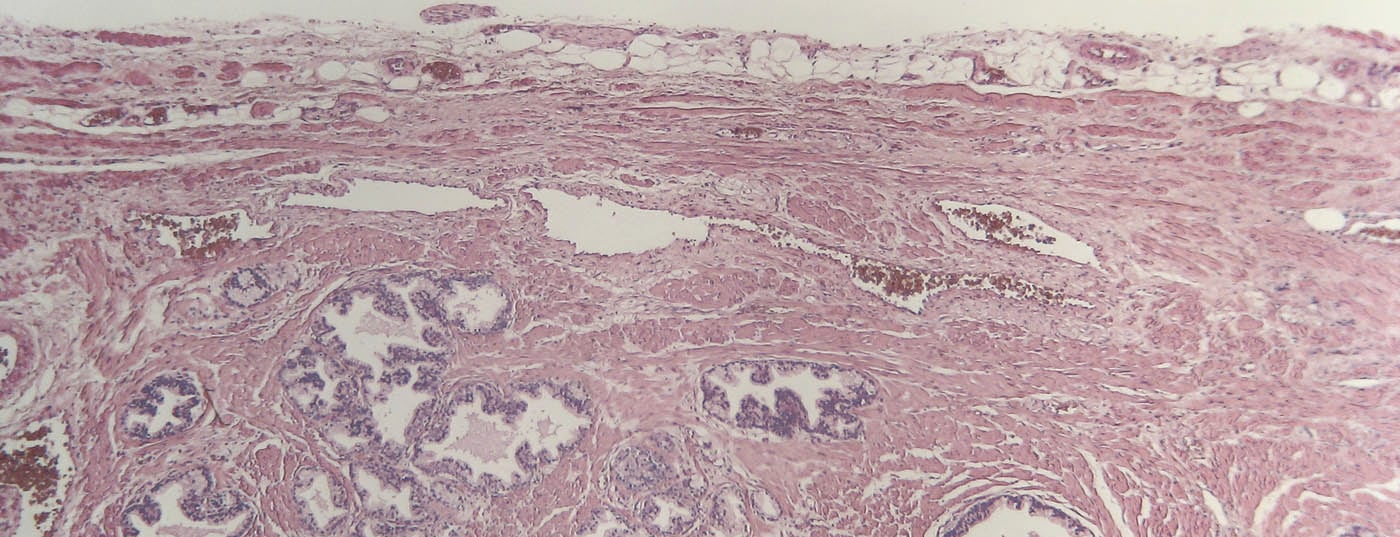
The term brachytherapy is derived from the Greek word “brachys” (near/short) because the radiation sources are placed directly in the tumor-bearing organ. The rice grain-sized implants (seeds) contain a radioisotope (iodine-125 or palladium-103) encased in a titanium capsule. Depending on the size of the prostate, usually 30-70 such seeds are inserted into the prostate, where they remain for life. The insertion is performed percutaneously from the perineum via 15-30 hollow needles (Fig. 1-3). The iodine-125 used in Switzerland emits low-energy X-rays and has a half-life of about 60 days. The procedure is performed under general anesthesia; hospitalization is short at three days and the patient is quickly able to return to work. Special radiation protection precautions are not necessary, since the dose rate measurable outside the patient is far below the maximum permissible value. Planning and execution of LDR brachytherapy are performed in interdisciplinary collaboration between urologists, medical physicists and radio-oncologists.
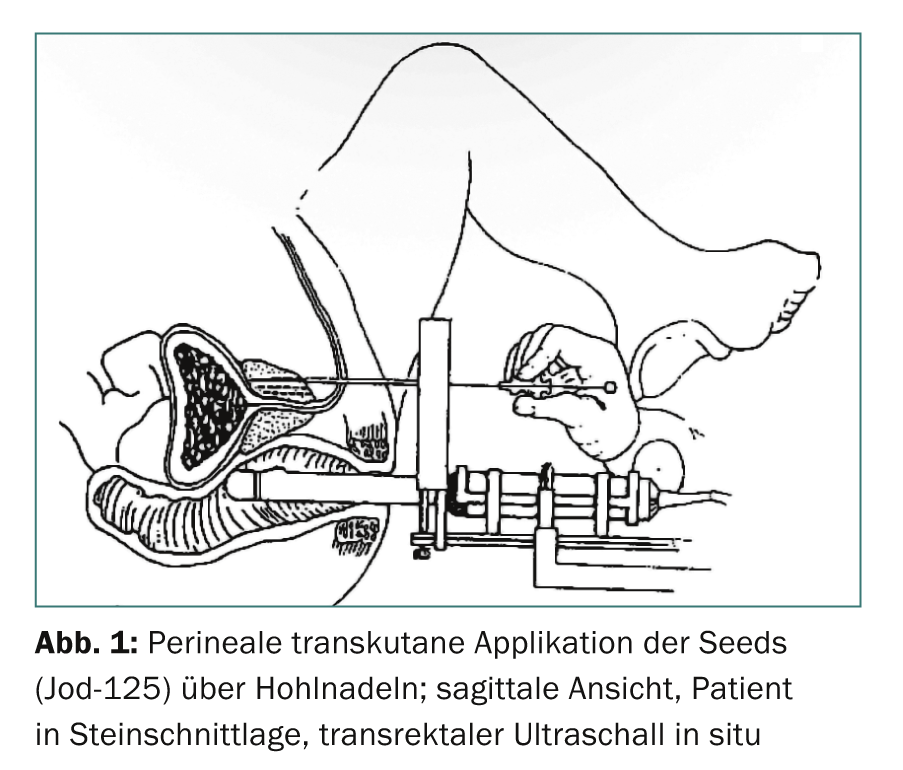
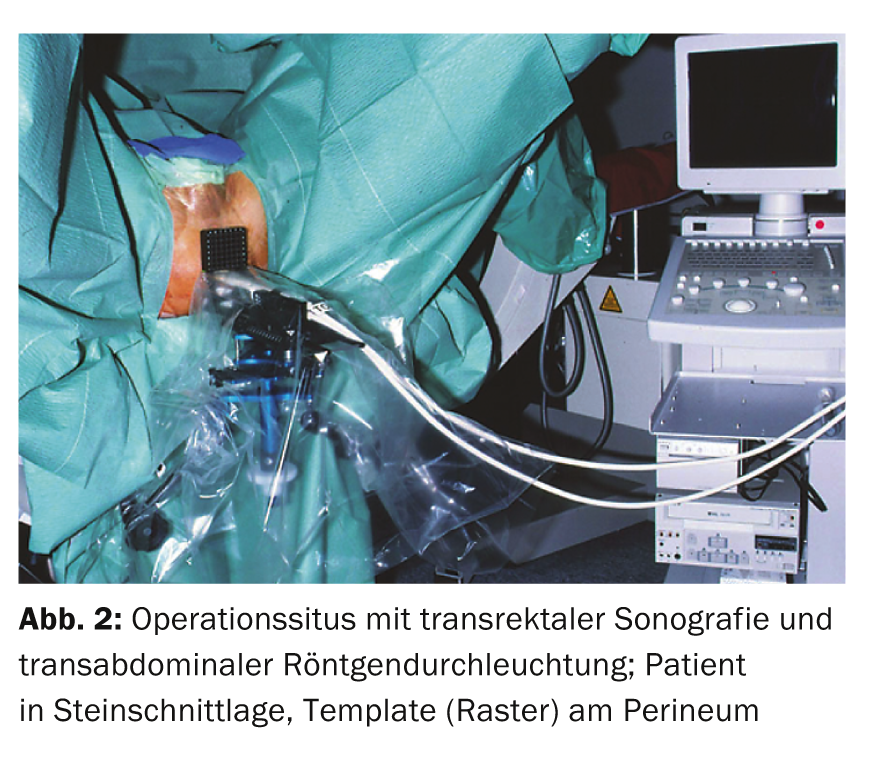
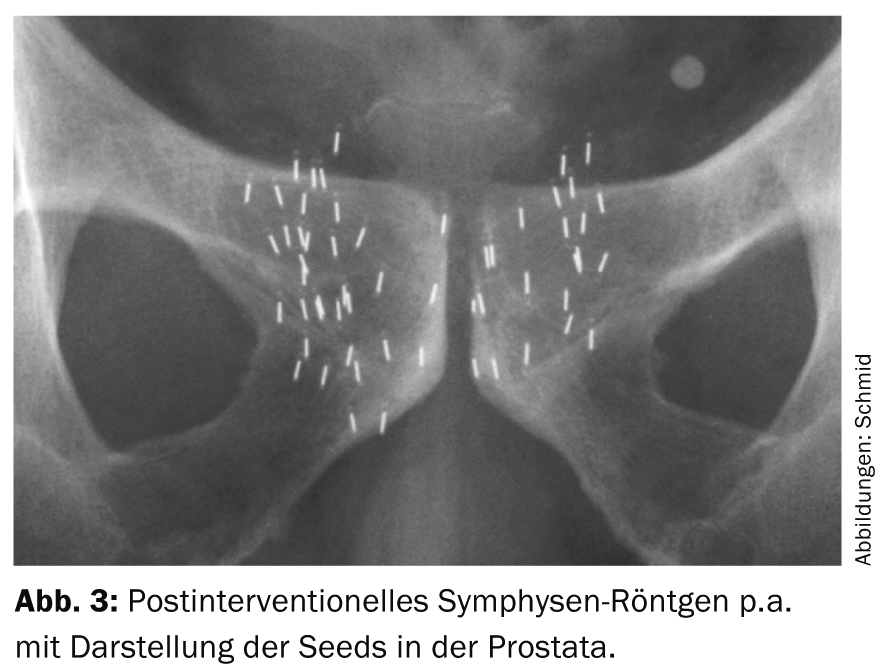
Indications
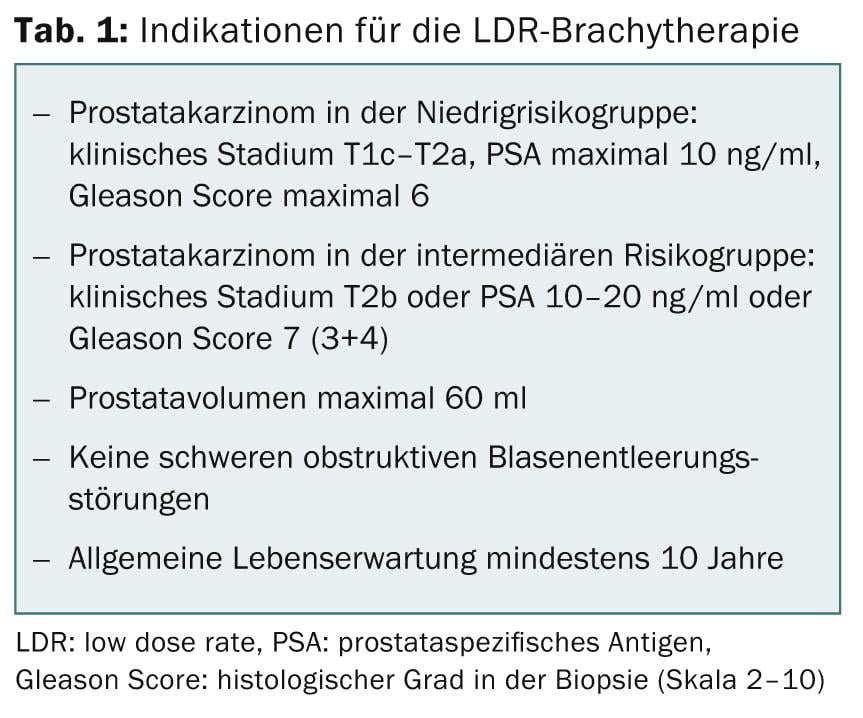
Due to the widespread determination of prostate specific antigen (PSA), the majority of prostate carcinomas are nowadays detected at an early stage and thus also qualify for seed implantation (Tab. 1). Modern brachytherapy has been used in the USA since 1985 and is becoming increasingly important. LDR brachytherapy has been a mandatory health insurance benefit in Switzerland since January 1, 2005. A condition for reimbursement is the prospective collection of all data on the basis of a detailed protocol.
Swiss register
The Federal Office of Public Health (FOPH) in Bern has designated the Cantonal Hospital of St. Gallen as the coordination center for recording and evaluating all brachytherapies performed in Switzerland since the beginning of 2005. As of June 2014, 1294 patients had been treated (mean age 64 years, range 43-82 years). Data collection will occur immediately preintervention and thereafter at 6 weeks and 6, 12, 24, 36, and 84 months after seed implantation. The main focus is on side effects and quality of life. The patient independently completes three questionnaires at the specified time points: “QLQ-C30” of the EORTC, “International index of erectile function” (IIEF) and “International prostate symptom score” (IPSS). In addition, “Adverse Events” (version 3.0), medications, urinary flow rate and post-micturition residual urine, degree of continence and number of any presentations as well as serum PSA are recorded. Dosimetry data will be reviewed once after six weeks using the image fusion method (MRI and CT). If there is an insufficient dose distribution, the “cold areas” can be re-spiked in a second session, but this occurs in only 2.3% of cases [4].
Oncological efficacy
The two most important and largest urological societies – “European Association of Urology” (EAU) and “American Urological Association” (AUA) – have published their revised guidelines on prostate cancer [1,2]. The methodology was different, but the conclusions regarding treatment of clinically localized prostate cancer are largely identical.
In a costly and time-consuming analysis lasting several years, the American experts assessed all 592 articles in English from 1991-2004 that provided oncologic results on the topic of treatment of T1-T2 prostate cancer. PSA recurrence-free survival at seven years, stratified by risk groups, was not different in the three treatment arms of prostatectomy, brachytherapy, and external beam radiotherapy. Since biochemical recurrence is very rare after this follow-up period, these data are unlikely to change even with prolonged observation [5].
The EAU guidelines cover a wider range and are more practice-based, therefore less rigorous in the scope of data analysis. The bottom line, as with AUA, is that radical prostatectomy, LDR brachytherapy, and external beam radiotherapy achieve comparable long-term oncologic outcomes, and no therapy is superior or inferior to another in this regard [6,7].
Side effects
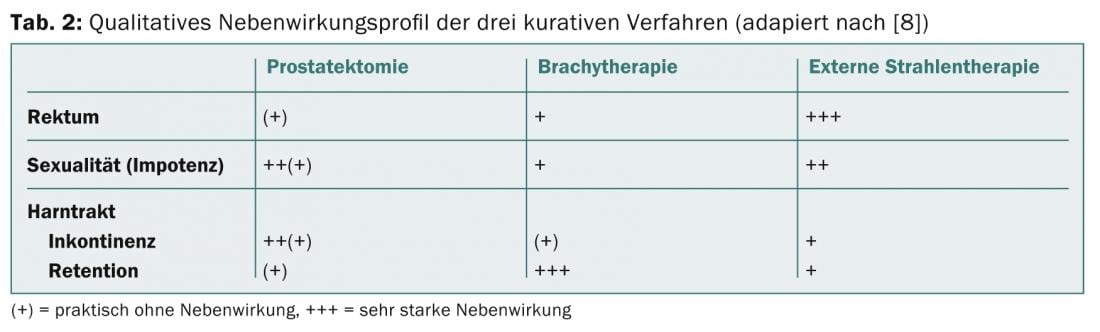
The three foregrounded adverse effects for all treatment options involve the urinary bladder, erectile function, and rectum. Table 2 presents a qualitative overview of side effects based on a structured literature review [8]. Main side effects of brachytherapy are obstructive micturition problems, comparable to those in benign prostatic hyperplasia. The causes are an increase in edema and volume due to the seeds. Symptoms may persist for several months, and most patients require a temporary α-1 receptor blocker. In rare cases, even trans-urethral resection of the prostate is indicated later. Regarding erectile function, the rule of thumb is that after five years, 50% of patients are still potent, while the other half respond well to phosphodiesterase inhibitors (sildenafil, tadalafil, vardenafil). There tends to be less complete erectile dysfunction after brachytherapy than after the other two therapies. However, no scientific proof can be derived from this.
Health-related quality of life after radical retropubic prostatectomy or LDR brachytherapy was retrospectively assessed using questionnaires at our hospital [9]. After a mean follow-up of 24 months (5-53 months), patients assessed global health status after surgery with
78 out of 100 points and after brachytherapy with 83 out of 100 points. This difference was not statistically significant. Similar conclusions were reached in a larger, prospective series from the United States [10].
Take-Home Messages
- LDR brachytherapy, percutaneous radiotherapy, and radical prostatectomy are the three curative therapies for localized prostate cancer recognized by all guidelines.
- Biochemical freedom from recurrence (no PSA rise) is nearly identical for all three procedures over the long-term.
- Because of the different side effect profiles, careful patient selection and unbiased, individualized counseling are important.
- Long-term experience with LDR brachytherapy in Switzerland confirms the good results of the international series.
Prof. Dr. med. Hans-Peter Schmid
Ladislav Prikler, MD
Literature:
- Heidenreich A, et al: EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 2011; 59 (1): 61-71.
- Thompson I, et al: Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 2007; 177(6): 2106-2131.
- Prikler L, et al: Brachytherapy of localized prostate carcinoma: a new treatment procedure in Switzerland. Schweiz Med Forum 2003; 3: 765-767.
- Putora PM, et al: Re-implantation after insufficient primary 125-I permanent prostate brachytherapy. Radiat Oncol 2013; 8(1): 194.
- Peinemann F, et al: Permanent interstitial low-dose rate brachytherapy for patients with localised prostate cancer: a systematic review of randomised an nonrandomised controlled clinical trials. Eur Urol 2011; 60: 881-893.
- Sylvester JE, et al: Fifteen-year biochemical relapse-free survival, cause-specific survival, and overall survival following I(125) prostate brachytherapy in clinically localized prostate cancer: Seattle experience. Int J Radiat Oncol Biol Phys 2011; 81(2): 376-381.
- Taira AV, et al: Long-term outcome for clinically localized prostate cancer treated with permanent interstitial brachytherapy. Int J Radiat Oncol Biol Phys 2011; 79(5): 1336-1342.
- Jani AB, et al: Early prostate cancer: clinical decision-making. Lancet 2003; 361: 1045-1053.
- Wyler SF, et al: Health-related quality of life after radical prostatectomy and low-dose-rate brachytherapy for localized prostate cancer. Urol Int 2009; 82 (1): 17-23.
- Sanda MG, et al: Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008; 358 (12): 1250-1261.