The goal of rheumatoid arthritis (RA) therapy today is clinical remission. The earlier the disease is detected, the better the chances of achieving this goal. For the road ahead, EULAR has provided an update on its recommendations for the management of RA with DMARDs.
The new EULAR treatment recommendations were posted online in November 2022 and have been available in print since March of this year [1]. They initially advise methotrexate (MTX) in phase 1 of therapy, provided there are no contraindications (desire to have children, pregnancy, lactation, renal or hepatic impairment). Conventional DMARDs such as leflunomide and/or sulfasalazine are available as alternatives. The goal is to achieve remission in the first 3-6 months.
If this fails, biologic DMARD therapy may be used, especially in cases with poor prognostic factors. Poor prognostic factors in RA include: high rheumatoid factors or AGPA antibodies, high levels of disease activity, i.e., high levels of inflammation or many swollen joints, and early erosive changes detectable by imaging. Previous 2020 guidelines advised bDMARDs or JAK inhibitors in such cases. In the update, this was changed to continue to recommend a bDMARD but to recommend the use of a JAKi “only after risk assessment.”
“When we start RA patients on a JAK inhibitor, we have to be careful especially in people >65 years of age, as well as if they smoke, have cardiovascular risk factors, diabetes, hypertension, or obesity, and have had or have a history of underlying malignancy or thromboembolic events,” explained Dr. Sarah Ohrndorf of the Department of Medicine with SP Rheumatology and Clin. Immunology, Charité – Universitätsmedizin Berlin (D) [2]. If clinical remission was still not achieved with the above options, either the bDMARD could be changed or a JAKi could be used in the following phase 3.
Reduce and taper glucocorticoids
Also new in the updated EULAR recommendations is the wording that a dose reduction of ongoing baseline antirheumatic therapy may be considered. However, it is important to note that glucocorticoids must be discontinued first. “If I have a diagnosis of RA, unless there are contraindications, I give MTX and glucocorticoids together, but the glucocorticoids are always just bridging therapy and should be reduced and stopped as soon as possible as the disease progresses,” Dr. Ohrndorf said. “Then, if sustained remission has been achieved without glucocorticoids, I may also consider reducing ongoing baseline therapy.”
With regard to comorbidities, the expert warns to always keep in mind that patients with inflammatory joint diseases such as rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis already have an increased cardiovascular risk per se compared to the normal population. These risk factors must be controlled accordingly. Therefore, EULAR recommends that a cardiovascular risk assessment be performed at least every 5 years. It should also be borne in mind that patients like to take NSAIDs such as ibuprofen on their own without consulting a doctor. It should therefore be pointed out in a conversation that this – especially if cardiovascular risk factors are already present – should only be taken with caution. In this context, it is also recommended to keep the dosages of glucocorticoid use as low as possible due to the spectrum of side effects.
DHPC for JAK inhibitors
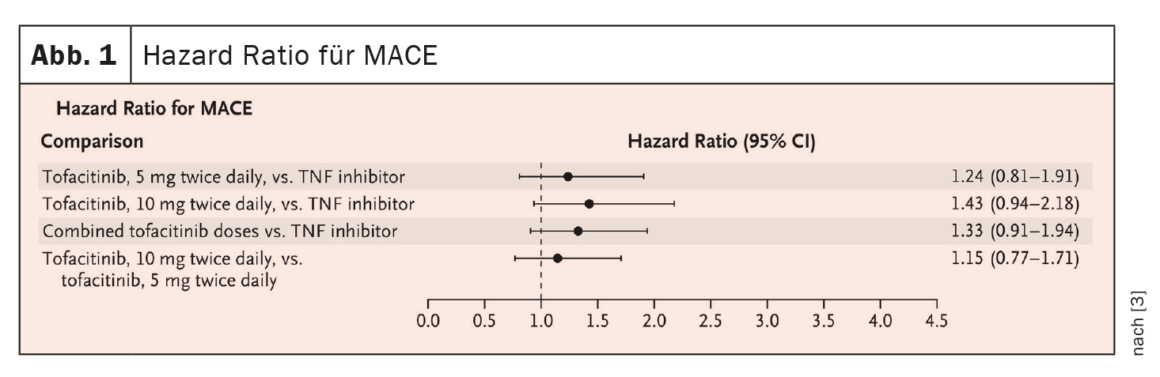
In RA, there are currently four approved JAK inhibitors: Baricitinib, tofacitinib, upadacitinib and filgotinib. In the ORAL Surveillance non-inferiority trial [3], RA patients ≥50 years with at least one cardiovascular risk factor were included and divided into three groups: Tofacitinib (TOF) 2x5mg/d, TOF 2x10mg/d, or a TNFi (adalimumab or etanercept) 2x/d, all in combination with MTX. As a result, for the primary comparison of combined TOF doses with TNFi, the noninferiority criterion was not met because the upper limit of the 95% CI of 1.94 was above the predefined noninferiority criterion of 1.8 ( Fig. 1).
On March 1, 2023, a DHPC (Direct Healthcare Professional Communications) infographic regarding increased risk for malignancies, serious cardiovascular events (MACE), serious infections, thrombosis, and all-cause mortality was published, “These risks are considered class effects and relevant to all approved JAK inhibitors for chronic inflammatory and dermatologic diseases” [4]. “From my point of view, this is somewhat problematic because the data on which this EMA recommendation is based come from the ORAL surveillance study – which was conducted solely with tofacitinib,” stated Jan Leipe, MD, Section of Rheumatology and Clin. Immunology, University Hospital Mannheim (D), to consider [2].
According to the EMA recommendation, JAK inhibitors should only be used in the following RA patients when no suitable treatment alternatives are available:
- Patients over the age of 65,
- Patients who currently smoke or have smoked in the past
- Patients with other risk factors for malignancies.
- Patients with other cardiovascular risk factors.
However, this recommendation must also be considered in relation, said Dr. Leipe: “If patients have previously failed conventional synthetic DMARDs or biologics, then in case of doubt there is no suitable alternative available at all. Then it is a matter of reducing the increased disease activity, since this is also a cardiovascular risk factor,” said the rheumatologist. In general, he said, the DHPC info does not mean that JAKi therapies are contraindicated if a corresponding risk factor is present, but “it is a recommendation that we should take into account and weigh in the treatment decision. If patients are very well controlled on JAK inhibitors, it’s very questionable whether you need to switch them.”
Finally, Dr. Leipe referred to a checklist for therapy with Janus kinase inhibitors published by the German Society of Rheumatology [5], which offers approaches on how to deal with the new prescription restrictions.
Take-Home Messages
- Remission is the therapeutic goal in RA
- RA treatment recommendations 2022 continue to suggest MTX as initial therapy
- New: bDMARDs or the use of JAKi are possible as second-line therapy, but the latter only under assessment of the risk profile (see DHPC).
- Third-line therapy is then again bDMARDs and JAKi on an equal footing
- Reduce or discontinue glucocorticoids as soon as possible, then consider de-escalation of baseline therapy.
- Patients with RA (or other inflammatory joint diseases) have an increased risk of CV, therefore:
- CV risk assessment min. Every 5 years
- General: Recommendation lifestyle adjustment, healthy diet, sufficient exercise, nicotine cessation.
- Prescribe NSAIDs under risk assessment (especially if CV risk factors are already known).
- Regularly review glucocorticoid dose, taper/discontinue in case of remission.
Congress: DGIM 2023
Sources:
- Smolen JS, Landewé RBM, Bergstra SA, et al.: EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis 2023; 82: 3–18; doi: 10.1136/ard-2022-223356.
- Sitzung WIN Rheumatoide arthritis. Was ändert sich durch neue Leitlinien in der Praxis. 129. Kongress der Deutschen Gesellschaft für Innere Medizin, 25.04.2023.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al.: Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med 2022; 386: 316–326;
doi: 10.1056/NEJMoa2109927. - www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/marktueberwachung/health-professional-communication–hpc-/dhpc-januskinase-jak-inhibitoren.html; last accessed 25.05.2023.
- https://dgrh.de/dam/jcr:a56b0f44-71b0-4278-8967-b2a7b6a2fd0f/Checkliste_JAKi.pdf.
InFo RHEUMATOLOGIE 2023; 5(1): 22–23