Prof. Dr. med. Claudia M. Witt, Zurich, and Dr. med. Christian Thuile, Merano, presented a pilot project at Merano Hospital at the 29th Swiss Annual Conference on Phytotherapy. In it, patients with breast carcinoma were studied in different therapy arms. Evaluation of the data provides evidence of the efficacy of additional complementary medicine treatment compared with conventional therapy alone.
(ag) According to Prof. Dr. med. Claudia M. Witt, Zurich, it is not always easy to draw conclusions from scientific evidence for everyday clinical practice. Many randomized trials evaluate only single measures in well-defined settings. Consequently, they often overlook the complex context in routine care and often include only highly selected patient groups. In order to be able to test the benefit of different therapies also in less standardized everyday conditions, with “typical” patients and by means of patient-relevant outcome parameters, the so-called Comparative Effectiveness Research (CER) was introduced in the USA.
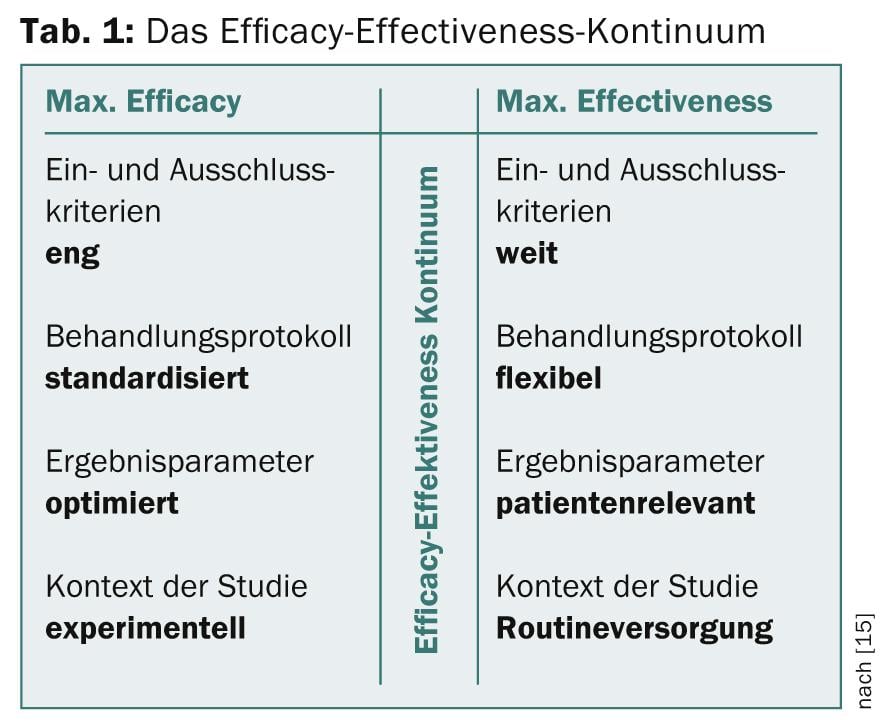
Most studies are on an efficacy-effectiveness continuum (Table 1) . While “efficacy” describes the extent to which a specific intervention is effective under ideal baseline conditions, “effectiveness” refers to the extent to which an intervention is effective for this population when applied in normal care.
Pilot project on complementary medicine
The study results of the pilot project initiated by the regional government of South Tyrol on the use of complementary medicine in patients with breast carcinoma were already presented in 2013 at a meeting of the International Society for Complementary Medicine Research (ISCMR) [1].
Under the direction of Dr. med. Thuile, a complex and individualized complementary medical therapy was applied. Among others, phytotherapy also played a crucial role (26% of patients received this intervention). It was offered symptom-related as an adjunctive therapy and aimed to minimize the side effects of conventional therapy as much as possible without inducing interactions. The study could not show whether any one of the procedures used was more effective than the other, but only whether complementary medicine (as applied at Merano Hospital) provided an overall additional benefit. Table 2 provides an overview of the medicinal plants frequently used at Merano Hospital and their indications.

A total of 275 patients were randomized, of whom 136 received additional complementary medicine and the others did not (n=139 in the control group). The primary endpoint (quality of life at six months) showed a statistically significantly greater improvement in the group receiving complementary medicine. Outcome was assessed using the FACT-B scale, a standard instrument used to assess quality of life in breast cancer patients. The value was 102.13 in the standard and 107.94 in the complementary group (p=0.0002) [1]. “An additional complex complementary medicine intervention was more effective in improving quality of life than routine care alone” concluded Prof. Witt in conclusion.
Source: “Complementary Medicine in Breast Cancer – Evaluation of the Pilot Project in Merano”, presentation at the 29th Swiss Annual Conference on Phytotherapy, June 18-21, 2004,
Winterthur
Literature:
- Witt C, et al: Effectiveness of an additional individualized multi-component complementary medicine treatment on health-related quality of life in breast cancer patients: A pragmatic randomized trial. Forsch Komplementmed 2013; 20(Suppl. 1): 45.
- May C, et al: Randomized open controlled clinical study on the efficacy and tolerance of an oral enzyme preparation in lymphadenectomy patients. Intern J Immunother 2001; 17: 149-152.
- Adamek J, Prausova J, Wald M: Enzyme therapy in the treatment of lymphedema in the arm after breast carcinoma surgery. Rozhl Chir 1997; 76: 203-204.
- Parikh PM, et al: Phlogenzym is safe and effective in reducing morbidity of vesicant chemotherapy extravasation. A prospective study. Intern J Immunother 2001; 17: 163-170.
- Kim JH, Park CY, Lee SJ: Effects of sun ginseng on subjective quality of life in cancer patients: a double-blind, placebo-controlled pilot trial. J Cl in Pharm Ther 2006; 31: 331-334.
- Barton DL, et al: Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support Care Cancer 2010; 18: 179-187.
- Mason L, et al: Systematic review of topical capsaicin for the treatment of chronic pain. BMJ 2004; 328: 991.
- Low PA, et al: Double-blind, placebo-controlled study of the application of capsaicin cream in chronic distal painful polyneuropathy. Pain 1995; 62: 163-168.
- Pommier P, et al: Phase III randomized trial of Calendula officinalis compared with trolamine for the prevention of acute dermatitis during irradiation for breast cancer. J Clin Oncol 2004; 22: 1447-1453.
- Manusirivithaya S, et al: Antiemetic effect of ginger in gynecologic oncology patients receiving cisplatin. Int J Gynecol Cancer 2004; 14: 1063-1069.
- Zick SM, et al: Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Support Care Cancer 2009; 17: 563-572.
- Biswal BM, Zakaria A, Ahmad NM: Topical application of honey in the management of radiation mucositis: a preliminary study. Support Care Cancer 2003; 11: 242-248.
- Motallebnejad M, et al: The effect of topical application of pure honey on radiation-induced mucositis: a randomized clinical trial. J Contemp Dent Pract 2008; 9: 40-47.
- Worthington HV, Clarkson JE, Eden OB: Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev 2006; 19: CD000978.
- Witt CM, Treszl A, Wegscheider K: Comparative Effectiveness Research: Tracking External Validity. Deutsches Ärzteblatt 2011; 108(46): A2468-2474.
- Witt CM, Thuile C: Complementary medicine in breast cancer – evaluation of the pilot project in Merano. Forsch Complementmed 2014; 21(suppl 1): 13-14.
InFo Oncology & Hematology 2014; 2(7): 28-29.