The epidemiology of multidrug-resistant bacteria has changed in Switzerland: significant decrease of MRSA and strong increase of ESBL-producing enterobacteria. ESBL germs can be acquired through food (poultry meat, vegetables, etc.). Travelers and patients repatriated from abroad are at increased risk for multidrug-resistant microorganisms. If there is no response to empirical antibiotic therapy, microbiological clarification is indicated due to possible resistance. E. coli including ESBL isolates are still well sensitive to fosfomycin and nitrofurantoin.
Increasing antibiotic resistance threatens many achievements of modern medicine. EU and U.S. health authorities each estimate about 25,000 deaths per year attributed to antibiotic resistance. Patients are unsettled by media reports of bacterial infections that are no longer treatable.
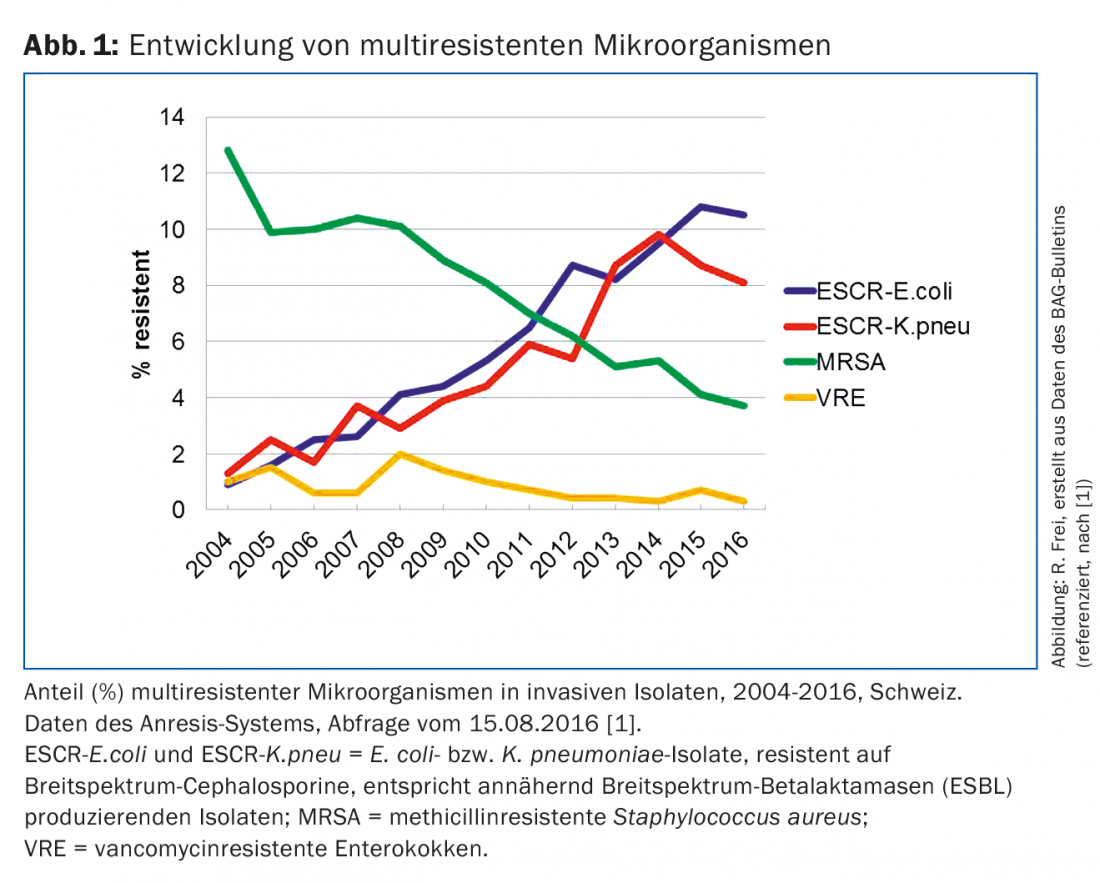
Multidrug-resistant bacteria are resistant to multiple classes of antibiotics. Multi-resistant microorganisms are causing major problems in hospitals worldwide, including various enterobacteria, Pseudomonas aeruginosa, Acinetobacter baumannii, Staphylococcus aureus and enterococci. Compared to many other countries, most resistance rates in Switzerland are lower. A primary care physician in this country is mainly confronted with multidrug-resistant Escherichia coli, less frequently with other resistant enterobacterial isolates and methicillin-resistant S. aureus (MRSA). Only occasionally does it come into contact with other multi-resistant germs, which include resistant campylobacter, gonococci and tuberculosis pathogens. According to the Swiss surveillance system Anresis (www.anresis.ch), the prevalence of multidrug-resistant bacteria has evolved differently over the past 12 years [1]. While the proportion of MRSA isolates has declined significantly, enterobacteria with resistance to broad-spectrum cephalosporins and other drug classes have increased steadily and sharply (Fig. 1) [1].
MRSA
In 2015, just under 6% of S. aureus isolates tested from outpatients in German-speaking Switzerland were still methicillin-resistant (MRSA). In southern and central European countries, on the other hand, the MRSA rate was significantly higher, for example 17% in France, 34% in Italy and as high as 47% in Portugal. In the past, MRSA spread mainly in hospitals and other medical institutions (healthcare-associated MRSA). For several years, new strains with different characteristics have emerged that can cause aggressive infections in the outpatient clinic even in younger patients without previous contact with the healthcare system (community-associated MRSA). In recent years, it has also been observed that individuals with close contact with animals have a higher risk of being MRSA carriers. MRSA could be found in various animals, especially horses and dogs, but particularly in farm animals such as chickens and pigs [2]. In Switzerland, the MRSA colonization rate in fattening pigs at the slaughterhouse has increased since 2009 from 2% to 26.5% in 2014. A clonal MRSA line (CC398), which is also commonly found in farm animals in other European countries, is responsible for this. It belongs to the “livestock-associated” MRSA, which particularly affects people with occupational contact with pigs, such as farmers and veterinarians [3]. Other people at risk for MRSA infections include individuals and travelers returning from southern Europe or the United States, patients and employees in healthcare facilities such as rehabilitation centers or nursing homes with elevated rates of MRSA.
Measures for MRSA
In cases of aggressive skin and soft tissue infections and/or lack of response to beta-lactam antibiotics (such as amoxicillin-clavulanic acid), MRSA should be suspected and microbiological diagnosis should be initiated. Carriers are best detected with a swab of the nose and throat. After patient contact in the doctor’s office, it is important to disinfect hands and contact surfaces with alcohol-based preparations. For closer contact, such as during an examination, gowns, face masks and gloves are useful. Inpatients should be contact isolated. Due to cross-resistance to penicillins and cephalosporins, all currently available peroral beta-lactam antibiotics are ineffective. Clindamycin, cotrimoxazole, or linezolid (Zyvoxid®) perorally are suitable as monotherapy in the outpatient setting if sensitivity has been demonstrated. Clindamycin should only be used if the strain has also tested sensitive to macrolides or MLSB resistance has been ruled out. For colonized immunosuppressed patients or healthcare workers, MRSA decolonization treatment can be performed after consultation with a center.
ESBL-producing Enterobacteriaceae
In Switzerland, 7% of Escherichia coli isolatesfrom the urogenital tract of outpatients are currently resistant to third-generation cephalosporins (Tab. 1) . Around 95% of these strains produce broad-spectrum beta-lactamases (ESBL), whose genes are mobile and localized on plasmids. This allows them to be transferred to other bacterial cells, species and genera and spread rapidly. These strains are often multidrug resistant.
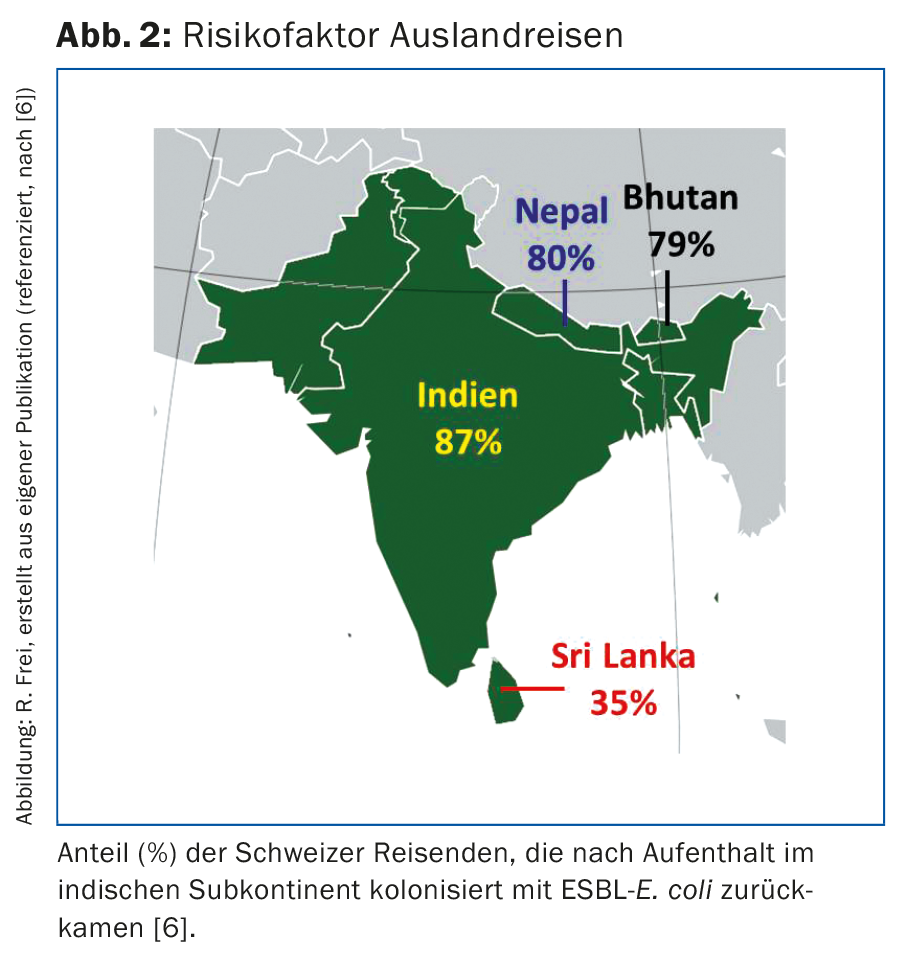
Before 2000, ESBL-producing E. coli and Klebsiella pneumoniae isolates were mainly found in patients in hospitals and nursing homes. Today, the majority of ESBL-E. coli are acquired outside of medical institutions. ESBL-producing bacteria can be found in various foods [2,4]. Thus, ESBL-E. coli were detected in 66% of chicken meat samples from Swiss production, and in chicken meat of foreign origin even in 86% of the samples [2]. Such strains were also found in samples of other poultry, pork and beef, as well as various vegetable samples. The use of antibiotics in veterinary medicine promotes the selection, multiplication, and spread of resistant bacteria in livestock and agriculture. In 2014, nearly 50,000 kg of antibiotics were sold in Switzerland for veterinary use [2]. In the U.S., four times more antibiotics are used for animals than for humans. Even if sufficient heating of the food kills the bacteria, germs can be transmitted to humans via contaminated kitchen utensils, hands and raw food. Our own investigations showed that 8% of kitchen boards in private households and 16% in hospital kitchens were contaminated with ESBL germs after processing chicken meat [5]. The gloves of the cooks were even ESBL-positive in 50%. Travel abroad poses a further risk: Swiss travelers to India were rectally colonized with ESBL-E. coli in 87% on return, after only 3% were ESBL carriers before departure (Fig. 2) [6]. At least a 30% carrier rate was found among travelers from other countries in the Indian subcontinent, Near and Southeast Asia, Africa, and South America [7]. Other individuals at increased risk for ESBL include slaughterhouse workers, cooks, nursing home residents, patients over 65 years of age, or those with antibiotic pretreatment [8].
Treatment of ESBL infections
ESBL-E. coli have also increased dramatically in Switzerland [1]. From 2004 to 2014, their proportion of invasive E. coli isolates increased tenfold (from 1 to 10%). Similar or higher rates have been reported in most European countries, with rates even exceeding 25% in Italy, Romania, and Bulgaria. The proliferation of ESBL-formers in all parts of Switzerland, in children as well as in adult and elderly patients, has made the therapy of E. coli and K. pneumoniae infections more difficult. Table 1 summarizes the current resistance of E. coli isolatesfrom outpatients. The table also shows multidrug resistance of ESBL isolates with resistance rates around 70% to quinolones and cotrimoxazole. In contrast, fosfomycin, nitrofurantoin, and carbapenems have low resistance rates between 0 and 4%, which can be used therapeutically for uncomplicated urinary tract infections. Therapy of other infections due to ESBL strains can be difficult and requires antibiotic resistance testing. For severe infections, carbapenems are considered the therapy of choice. Since ertapenem (Invanz®) only needs to be administered once daily (1 g i.v.), it can also be used in the outpatient setting. The clinical effect of amoxicillin-clavulanic acid is uncertain even when ESBL pathogen sensitivity is demonstrated, except in urinary tract infections. ESBL carriers are most reliably detected by rectal swab or stool specimen, urine, and specimens from clinically infected sites [8]. Unfortunately, however, no decolonization scheme to date has shown long-term success. As transmission of ESBL-E. coli has shifted from the hospital to the community and transmission rates in acute care hospitals are low, many acute care hospitals have moved away from strict contact isolation for ESBL-E. coli [8]. Contact isolation is still recommended for patients colonized or infected with other ESBL-producing enterobacteria (e.g., K. pneumoniae).
Nightmare carbapenemases
Today, more than 1000 different bacterial beta-lactamases are known. Among them, carbapenemases are the most feared, as they inactivate not only carbapenems but virtually all beta-lactam antibiotics. Most carbapenemase-producing Gram-negative rods are also extremely multidrug resistant. Even extended antibiotic resistance testing often shows only 1 to 2 active compounds, such as colistin. Sometimes none of the preparations available on the market is effective in vitro anymore. Such strains have occurred only sporadically in Switzerland and were almost always imported from other countries. KPC-type carbapenemases are clustered in Italy, Greece, Israel, and the United States and are the most common type in Switzerland to date (approximately 45% of identified carbapenemases) [9]. NDM-type carbapenemases have spread mainly in India and Balkan countries and have been carried to other countries with travelers, patients, and migrants. Enzymes of the OXA-48 group were introduced into European countries from Turkey, North Africa and the Middle East and account for approximately one third of the carbapenemases in Switzerland. Recently, a colistin resistance mechanism (MCR-1) was discovered in isolates from China and other countries including Switzerland that spreads with plasmids [10]. A practitioner caring for a patient with carbapenemase-producing bacteria would be advised to consult with an infectious disease department.
Literature:
- Federal Office of Public Health. Anresis.ch: Reports of selected multiresistant microorganisms in Switzerland. Bulletin 2016;(35): 530.
- Federal Food Safety and Veterinary Office FSVO: ARCH-Vet. Report on the distribution of antibiotics in veterinary medicine and antibiotic resistance monitoring in farm animals in Switzerland. General Report 2014. www.blv.admin.ch/blv/de/home/tiere/tierseuchen/tierarzneimittel/antibiotika/vertrieb.html
- Larsen J, et al: Evidence for human adaptation and foodborne transmission of livestock-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2016; Sep 20. pii: ciw532. [Epub ahead of print]
- Zogg AL, et al: Characteristics of ESBL-producing Enterobacteriaceae and methicillin-resistant Staphylococcus aureus (MRSA) isolated from Swiss and imported raw poultry meat collected at retail level. Schweiz Arch Tierheilkd 2016; 158(6): 451-456.
- Tschudin-Sutter S, et al: Extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae: a threat from the kitchen. Infect Control Hosp Epidemiol 2014; 35(5): 581-584.
- Kuenzli E, et al: High colonization rates of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli in Swiss travelers to South Asia – a prospective observational multicentre cohort study looking at epidemiology, microbiology and risk factors. BMC Infect Dis 2014; 14:528.
- Ostholm-Balkhed A, et al: Travel-associated faecal colonization with ESBL-producing Enterobacteriaceae: incidence and risk factors. J Antimicrob Chemother 2013; 68(9): 2144-2153.
- Tissot F, et al: Enterobacteriaceae with broad-spectrum beta-lactamases (ESBL) in hospitals: new Swissnoso 2014 recommendations. www.swissnoso.ch/wp-content/uploads/pdf/v18_2_de.pdf
- Babouee B, et al: Emergence of four cases of KPC-2 and KPC-3-carrying Klebsiella pneumoniae introduced to Switzerland, 2009-10. Euro Surveill 2011; 16(11). pii: 19817.
- Nordmann P, et al: Plasmid-mediated colistin resistance: an additional antibiotic resistance menace. Clin Microbiol Infect 2016; 22(5): 398-400.
HAUSARZT PRAXIS 2016; 11(12): 16-19