An inadequately adjusted blood glucose level can result in disorders of the somatic and/or autonomic nervous system – and more quickly than is commonly assumed!
Somatic and/or autonomic nervous system disorders are a common consequence of inadequately controlled blood glucose levels. A direct correlation between level of blood glucose, duration of hyperglycemia and development of neuropathy was demonstrated. Therefore, it can also occur in all forms of diabetes mellitus. Due to the disturbed metabolism in the nerve cell, glycated metabolites are stored in the nerve tissue. Microvascular changes of the nerve capillaries with occlusion of the lumen and thickening of the vessel walls entails a lack of oxygen, so that a gradual loss of function occurs. At the time of diagnosis of type 2 diabetes, peripheral nerve damage can be found in as few as 12% of those with the disease. The incidence increases linearly over time and averages 30%.
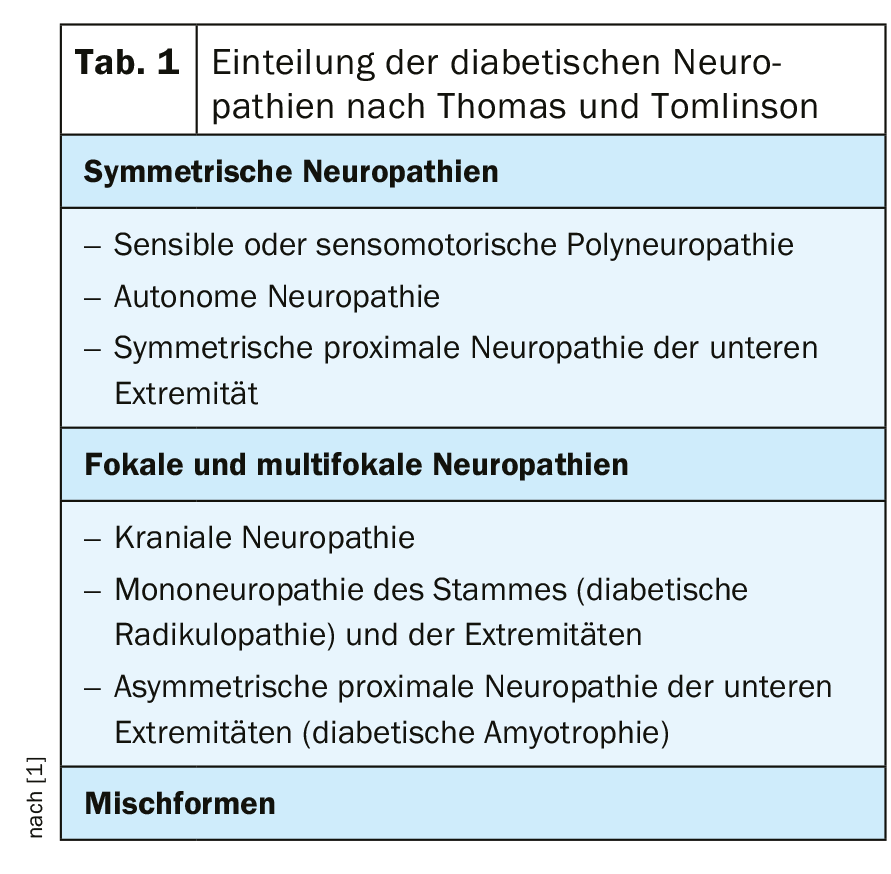
Diabetic neuropathies can be divided into sensorimotor and autonomic diabetic neuropathies. Their manifestation is classified according to clinical criteria (Table 1) [1].
The most common diabetic neuropathy is chronic distal symmetric polyneuropathy (DSPN), accounting for approximately 30% [1]. It is defined as the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after exclusion of other causes. The risk increases with an increase in other risk factors, indicators and comorbidities (box). However, up to half of all diseases can be asymptomatic, which then often go unrecognized and increase the risk of ulcers, for example. However, quality of life can also be severely affected by neuropathic pain. This is the case for up to 25% of all affected persons. Four clinical manifestations are distinguished:
- Subclinical (asymptomatic) neuropathy: no complaints or clinical findings, quantitative neurophysiological tests (vibratometry, quantitative thermesthesia, electroneurography) are pathological
- Chronic painful neuropathy (common): Painful symptoms at rest (symmetrical and increasing at night): Burning, shooting or stabbing pain, paresthesias, dysesthesias, numbness, unpleasant tingling, sleep disturbances; loss of sensation of varying quality, reduced muscle reflexes on both sides.
- Acute painful neuropathy (rare): Symmetric pain in the lower extremities and possibly also in the trunk area is prominent; Possibly additional hyperesthesia; Sensory disturbances in the lower extremities or normal neurological examination findings; May be associated with the start or intensification of insulin therapy (“insulin neuritis”).
- Painless neuropathy (common): Absence of symptoms or numbness and/or paresthesias; reduced or absent sensitivity, absent muscle intrinsic reflexes (especially Achilles tendon reflex), gait unsteadiness, unnoticed injuries or ulcers

Early diagnosis of polyneuropathy
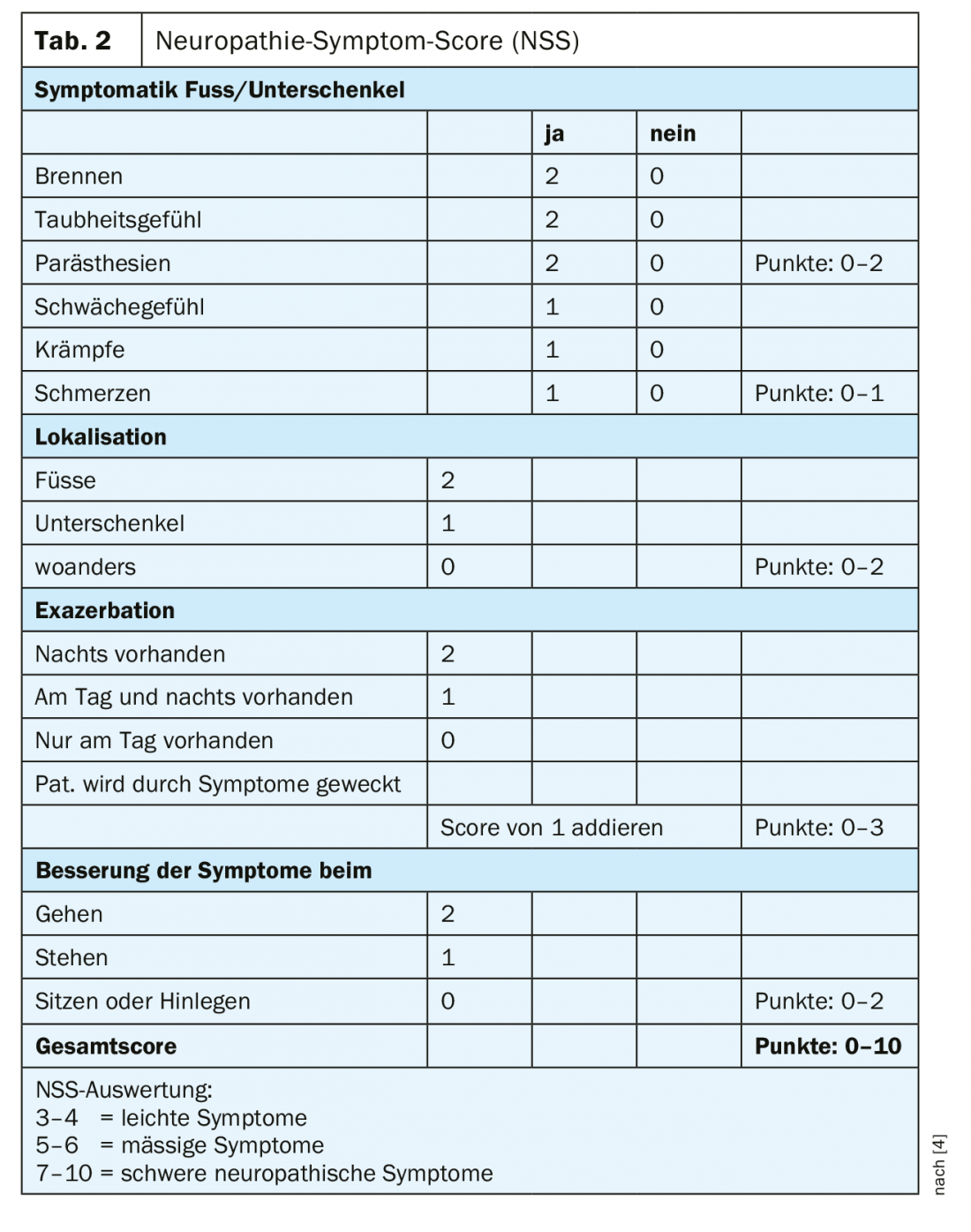
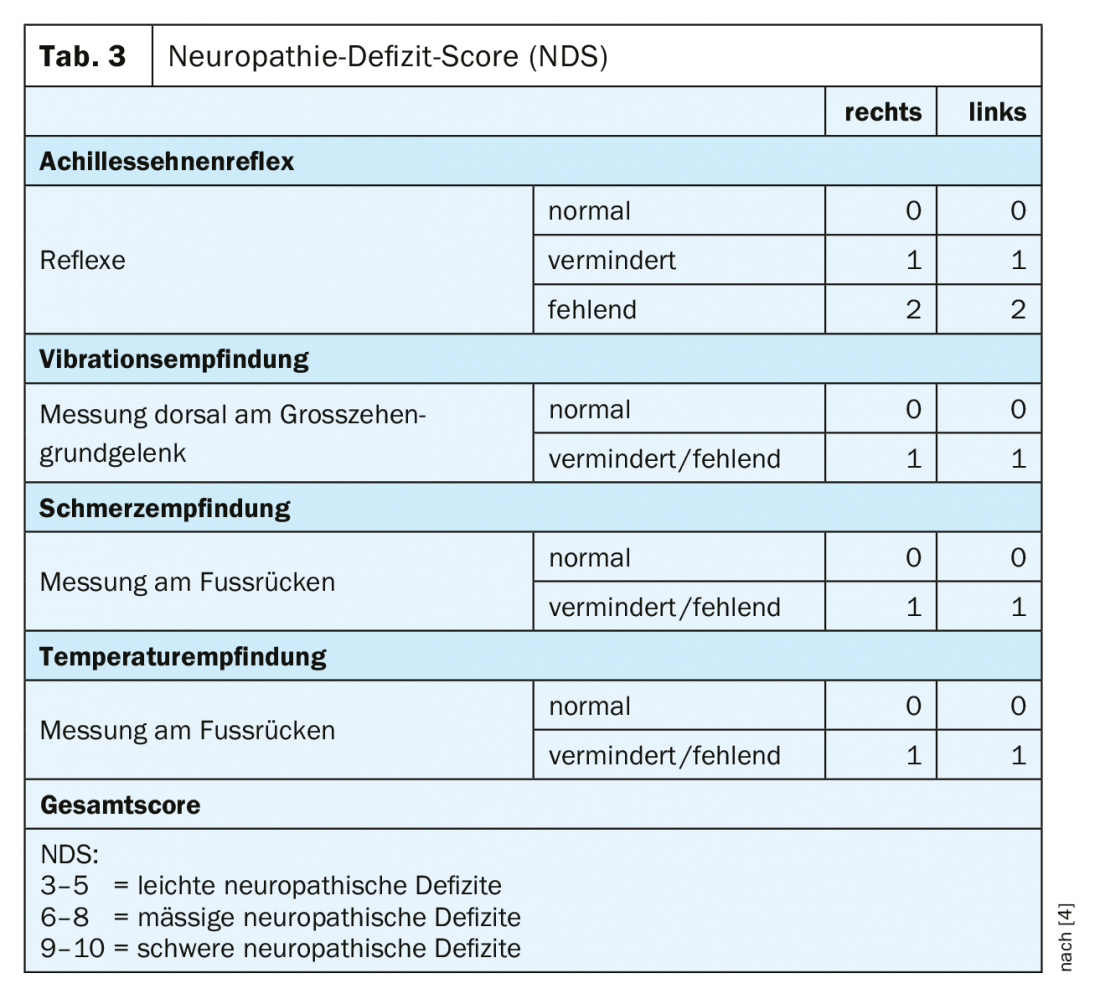
In practice, diabetic polyneuropathy is recognized much too late. Even in painful forms, almost 60% of those affected assume they do not suffer from a nervous system disorder [3]. This makes early diagnosis all the more important. Screening for sensorimotor diabetic polyneuropathy should include neuropathic plus and minus symptoms in addition to a history with basic personal data, diabetes-specific information, and the recording of risk factors. In addition, an inspection and clinical examination should be performed, screening for foot complications and peripheral arterial disease, and simple neurologic examinations. The neuropathy symptom score (NSS) can be used well for basic clinical diagnosis (Table 2) [4]. To assess the severity of neuropathy, it is useful to perform the neuropathy deficit score (NDS) (Table 3) [4]. An examination with regard to a possible neuropathy should be performed in patients with type 2 diabetes at the time of diagnosis and repeated annually thereafter, and in patients with type 1 diabetes at the latest five years after diagnosis.
A study of intraepidermal nerve fibers in patients with type 2 diabetes has shown that polyneuropathy is by no means just a concomitant disease of the later stage of the disease [5,6]. It could be shown that – despite good blood glucose control with an average Hba1c value of 6.5 – a decrease of intraepidermal nerve fibers by about 20% occurred very early. A similar development can be seen in the nerve fibers in the cornea. Already in the first year, both fiber length and nerve fiber density decrease [7]. By means of confocal corneal microscopy (CCM) these developments can be easily detected.
However, not every polyneuropathy associated with diabetes mellitus has to be a diabetic polyneuropathy. Therefore, a minimal internal medicine program with the laboratory parameters blood count, creatinine, ESR, TSH, vitamin B12, folic acid, alanine aminotransferase (ALAT), gamma-GT and immunoelectrophoresis (paraproteinemia) is recommended for differential exclusion. If there is no evidence of pathologic values, a neurologist should be consulted for clarification.
A neurologist should generally be consulted if one or more of the findings apply [1]:
- Predominance of motor rather than sensory deficits,
- rapid development and progression of symptoms,
- severe asymmetry of neurological deficits, mononeuropathy and cranial nerve disorder,
- Progression of symptoms despite optimization of the metabolic situation,
- Onset of symptoms in the upper extremities,
- Evidence of other neurologic symptoms beyond diabetic polyneuropathic syndrome,
- Family history of neuropathy.
Three-column treatment regime
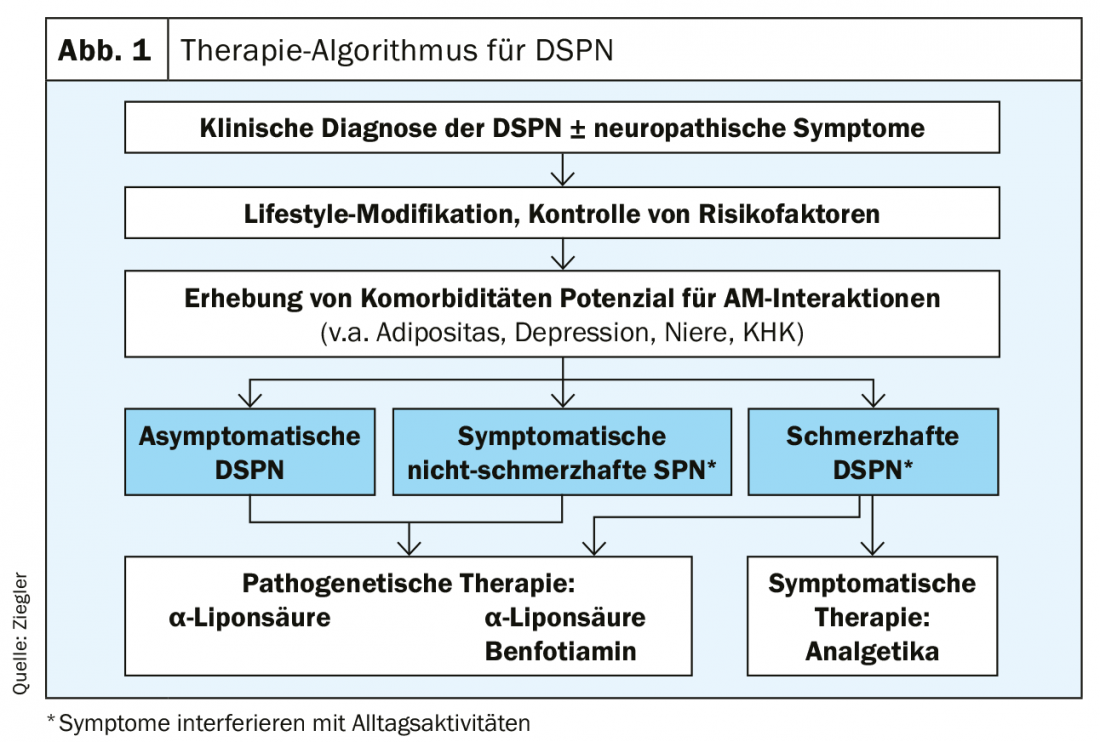
The therapy of diabetic neuropathy is essentially based on three pillars. The focus is on optimal diabetes control, which includes lifestyle adjustment and multifactorial intervention. Pathogenetic therapy is most effective in asymptomatic or non-painful DSPN. Symptomatic pain management can significantly improve the quality of life for those with painful DSPN. The combination therapy of duloxetine and pregabalin is useful here (Fig. 1).
If blood glucose is lowered too rapidly and too significantly at baseline in newly diagnosed diabetics, therapy-induced neuropathy (TIND) may occur [8]. Especially if the HbA1c level is lowered above 7%, acute painful neuropathy may occur after weeks or months. This regresses after about a year, but requires intensive treatment with analgesics. The risk can be significantly reduced by lowering the HbA1c level by an average of two to three points in three months.
To specifically interfere with the metabolic pathways of hyperglycemia-induced microvascular damage, α-lipoic acid, benfotiamine, and actovegin can be used. For α-lipoic acid, the dose should be 600 mg to achieve a good effect [9]. This can be demonstrated over a four-year period [10]. Benfotiamine 600 mg also produced a beneficial effect on neuropathy symptoms [11]. After six weeks, the NSS improved significantly.
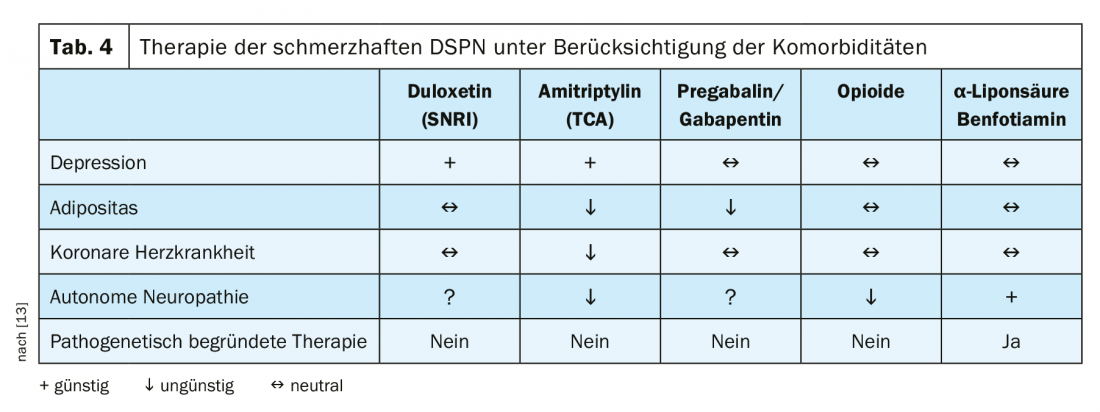
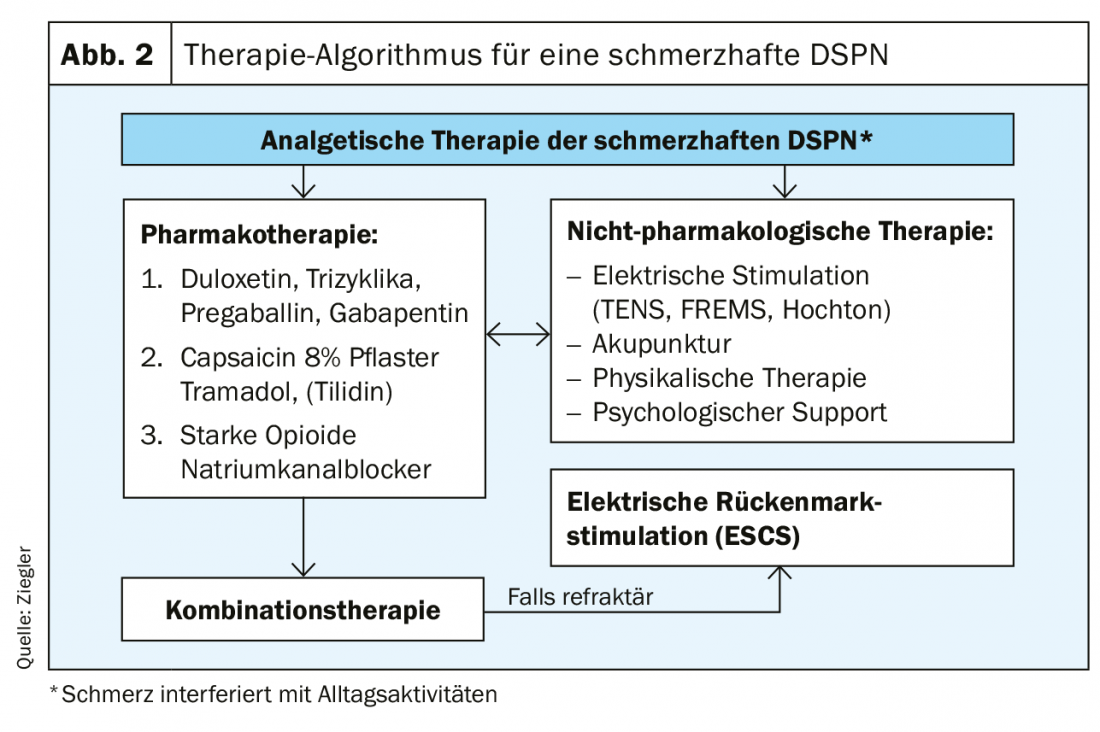
Otherwise, the therapy can be built up mainly symptomatically. These include recording pain, mood, functionality, and sleep. Because pain is subjective, a Numeric Pain Rating Scale (NRS) should be used for assessment. Regarding the evidence of pain therapies, a moderate strength of evidence was found for SNRI. Anticonvulsants, tricyclic antidepressants, atypical opioids, alpha-lipoic acid, and electrical spinal cord stimulation have low evidence [12]. In addition, therapy should also take comorbidities into account. Thus, a predamaged heart is a contraindication for nonselective monoamine reuptake inhibitors (Table 4) [13]. The therapy algorithm shown in Figure 2 once again clearly presents all steps of analgesic therapy for painful DSPN.
Take-Home Messages
- Diabetic neuropathy often occurs as a result of diabetes disease. Contrary to the common belief that it manifests itself only as a late consequence in the longer course of time, a decrease in nerve fibers could already be observed in the first year after diagnosis.
- Chronic distal symmetric polyneuropathy is the most common form of diabetic neuropathy (DSPN) and can be divided into symptomatic nonpainful DSPN, painful DSPN, and asymptomatic DSPN.
- Therapy depends on the severity and possible comorbidities. Good management of the underlying disease is obligatory. In addition, there is pathogenetic as well as symptomatic treatment.
- In addition to SNRIs, anticonvulsants, tricyclic antidepressants, capsaicin patches, and opioids are available for pharmacologic pain management in DSPN.
Literature:
- NVL Neuropathy in Diabetes in Adulthood. www.awmf.org/uploads/tx_szleitlinien/nvl-001e_k_S3_Diabetes_Neuropathie_2016-08.pdf (last accessed on Jun 12, 2019)
- Pop-Busui R, et al: Diabteic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017; 40: 136-154.
- Ziegler D, et al: Painful and painless neuropathies are distinct and largely undiagnosed entities in subjects participating in an educational initiative (PROTECT study). Diabetes Res Clin Pract 2018; 139: 147-154.
- Ziegler D et al: Diabetic neuropathy. Diabetology, 2018; 13: 230-243.
- Strom A, et al: Pronounced Reduction of Cutaneous Langerhans Cell Density in Recently Diagnosed Type 2 Diabetes. Diabetes 2014; 63: 1148 1153.
- Ziegler D, et al: Overexpression of cutaneous mitochondrial superoxide dismutase in recent-onset type 2 diabetes. Diabetologia 2015; 58: 1621-1625.
- Ziegler D, et al: Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes 2014; 63: 2454-2463.
- Gibbons CH, Freeman R: Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain 2015; 138: 43-52.
- Amato Nesbit S, et al: Non-pharmacologic treatments for symptoms of diabetic peripheral neuropathy: a systematic review. Curr Med Res Opin 2018; 17: 1-11.
- Ziegler D, et al: Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care 2011; 34: 2054-2060.
- Stracke H, et al: Benfotiamine in diabetic polyneuropathy (BENDIP): results of a randomised, double blind, placebo-controlled clinical study. Exp Clin Endocrinol Diabetes 2008; 116: 600-605.
- AHRO: Preventing Complications and Treating Symptoms of Diabetic Peripheral Neuropathy. Comparative Effectiveness Review, Number 187, 2017.
- Ziegler D: Painful diabetic polyneuropathy. Neurology 2012; 31: 140-146.
HAUSARZT PRAXIS 2019; 14(7): 8-11
CARDIOVASC 2019; 18(5): 16-19