Enlarged neck lymph nodes that persist for more than two weeks must be clarified. Ultrasound and fine needle aspiration are the gold standard. Open biopsies are initially contraindicated! Slice imaging should be ordered directly by the clinic that will later treat the patient or in consultation with the physicians responsible there. This avoids unnecessary costs. With higher T- and N- stage (primary tumor and lymph node status) the probability of distant metastases increases. Here, a PET-CT scan can usefully complement slice imaging for surgery or radiation planning.
Head and neck tumors are the sixth most common tumor entity in Switzerland and worldwide. Approximately 90% are more or less well differentiated squamous cell carcinomas.
While the incidence of noxious-induced carcinomas is slowly decreasing in our country, the incidence of HPV-induced tumors of the pharynx (mainly tonsil and tongue base) is rapidly increasing. These have a much better prognosis (at the same tumor stage) compared to tumors induced by noxious agents [1]. This has long been attributed to the increased radiosensitivity of HPV-induced carcinomas, but this does not appear to be true. In a nationwide multicenter study, patients with HPV-induced tumors who were treated “only” surgically also had a better prognosis than HPV-negative patients [2]. In general, depending on etiology (noxious agents, HPV, both, none) and tumor stage at diagnosis, prognosis is approximately 50-70% long-term survivorship.
Symptomatology and diagnostics
Most patients come to the doctor with one or more painless lumps on the neck. It is important to think of a tumor in the oropharyngeal region when indolent cervical lymph nodes are present; not infrequently, primary tumors are small (typically in HPV tumors) and asymptomatic or “hiding” in sites that are not easily accessible (tonsillar crypts, base of tongue, posterior floor of mouth, etc.).
The investigations of choice in this regard are ultrasound and fine needle aspiration. Open biopsies are contraindicated for several reasons: There is a non-negligible risk of tumor spread to the soft tissues of the neck by opening the lymph node metastasis, and open biopsies compromise the subsequent second surgery in the sense that the risk for complications increases. Many studies have shown that fine-needle aspiration does not pose a risk with regard to carryover of tumor cells [3].
Based on the size and extent of the primary tumor and the size and number of affected lymph nodes, the tumor stage is determined. In Switzerland, too, the UICC/AJCC international standard applies to this [4]. If one assumes that a tumor is induced by noxious substances (nicotine and/or heavy alcohol consumption), a so-called field carcinogenesis is present. This means that genetic damage has occurred not only at the site of the tumor, but potentially in the entire upper aerodigestive tract. In this situation, it is important to exclude synchronous second tumors, which occur with an incidence of 2-8%. Although performing a panendoscopy (mirroring of the entire upper airway and esophagus) is considered standard for this purpose, with today’s better quality of slice imaging (CT, MRI, PET-CT, more recently PET-MR), it is also possible to dispense with such an examination with additional anesthesia [5]. If surgery is planned, however, sometimes only examination under anesthesia can provide clarity as to whether and how a tumor is operable; fecal examination is not always conclusive due to severe gag reflex, poor compliance, and pain.
For a complete workup, most tumors (except the very small ones) always require slice imaging. If surgery is likely, MRI is usually the examination of choice in the oral cavity and pharynx. For laryngeal malignancies, contrast CT will be the primary choice because there are more swallowing artifacts when performing an MRI due to the longer acquisition time. If the likelihood of distant metastases increases due to a high T or N stage (T3,4; N2b,N2c,N3), it is recommended that PET-CT be performed to rule them out.
If an “outline” with clinical examination, cyto- and/or histopathological diagnosis, exclusion of second carcinomas and distant metastases as well as tomographic imaging is available, the presentation takes place at the interdisciplinary tumor board, where the therapy plan is determined. Curative therapeutic approaches include surgery, radio (chemo) or radio (immuno) therapy, or a combination of both. A small percentage of patients are no longer amenable to curative treatment due to tumor size, distant metastases, or patient-related factors; these are most often treated with palliative chemotherapy or immunotherapy.
Surgical therapy of the primary tumor
If a tumor is located in the oral cavity (tongue, floor of the mouth, gingiva, cheek, hard palate), surgical therapy will be chosen whenever possible, as the success of primary radiation in this area is disproportionately low in the curative setting (cure rate approx. 30-40%) [6]. Depending on the size of the resection defect, reconstruction with a tissue transfer in the form of a free flap (radial, femoral, fibular flap, etc.) may be required. In this procedure, a flap corresponding to the defect is lifted, sutured into the area of the former tumor, and its vessels are connected to the neck vessels by microsurgery.
If a tumor is located in the oropharyngeal region, the expected postoperative functionality usually determines whether surgery is appropriate. If a tumor involves, for example, large portions of the soft palate or the base of the tongue, problems that should not be underestimated are to be expected even after careful reconstruction, e.g., nasal reflux, complete nasal obstruction (obstructive sleep apnea syndrome), or difficulty swallowing with aspiration; in this case, primary radiochemotherapy is more likely to be chosen.
In the case of tumors in the hypopharynx that spread to the larynx, a laryngopharyngectomy may be necessary, especially in the setting of salvage surgery (rescue surgery in the case of unsuccessful radiation); in this case, the entire pharyngeal mucosa and muscles are removed along with the larynx. Here, too, reconstruction is performed using a tubular free flap (Fig. 1).
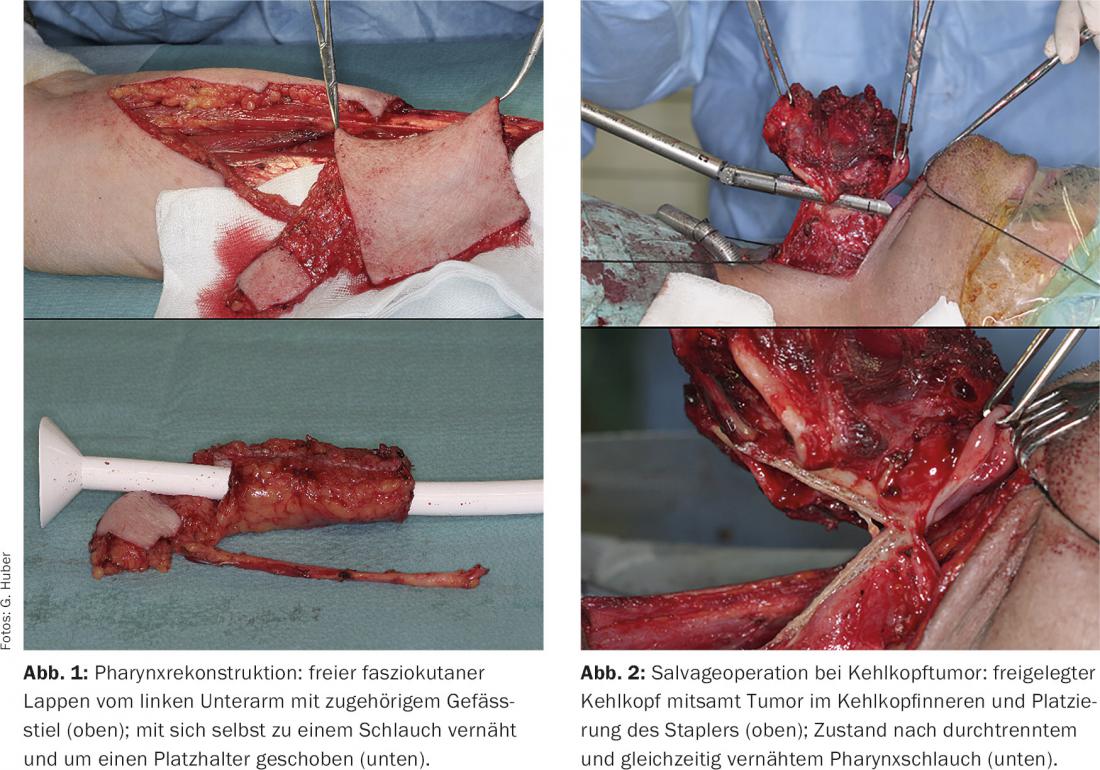
Small tumors of the larynx can usually be removed by laser without major loss of voice. For larger tumors, radiation will be preferred for functional reasons. If this is not successful, the only option is often complete laryngectomy, which can sometimes also be performed with a stapler (Fig. 2) . Postoperative voice and swallowing rehabilitation usually goes very well; fortunately, the days of “robotic voice” with the Servox device are over since implantable speech prostheses have become available.
Treatment of the cervical lymph nodes
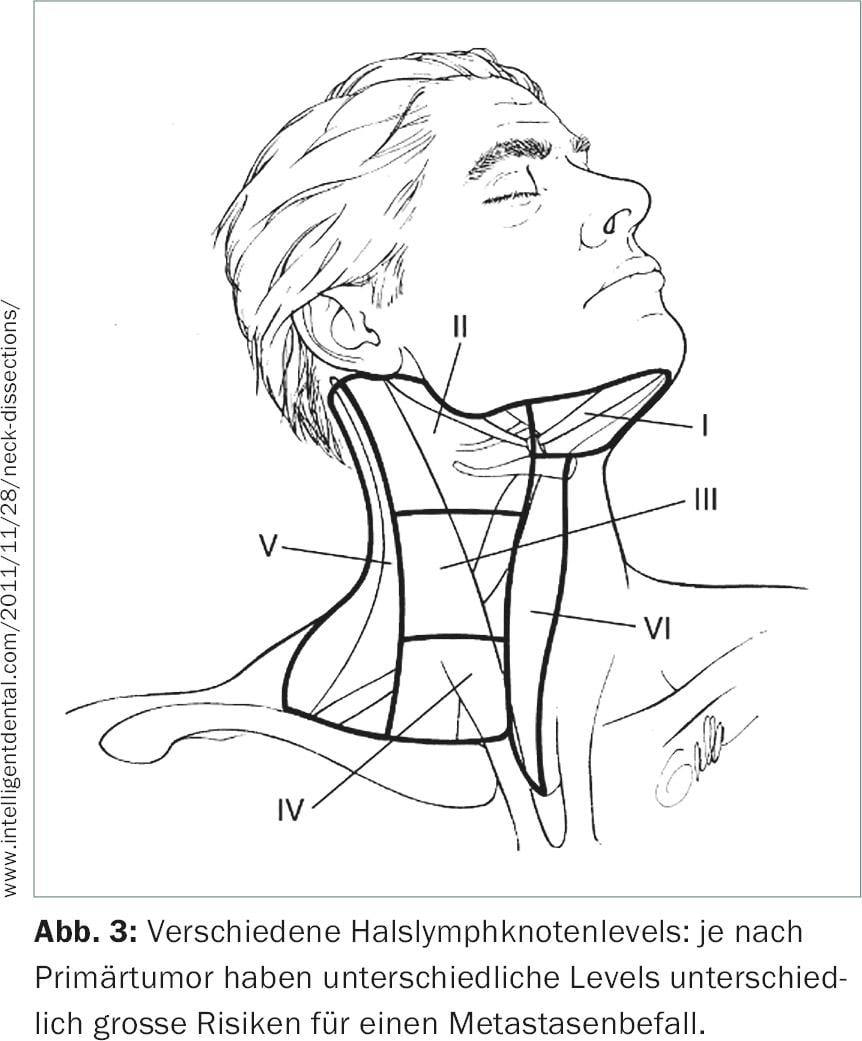
The treatment of the cervical lymph nodes is almost invariably part of the therapy concept. The neck is divided into six subregions, so-called levels (Fig. 3) . If lymph nodes are already affected, levels I-IV are usually removed completely (neck lymph node dissection).
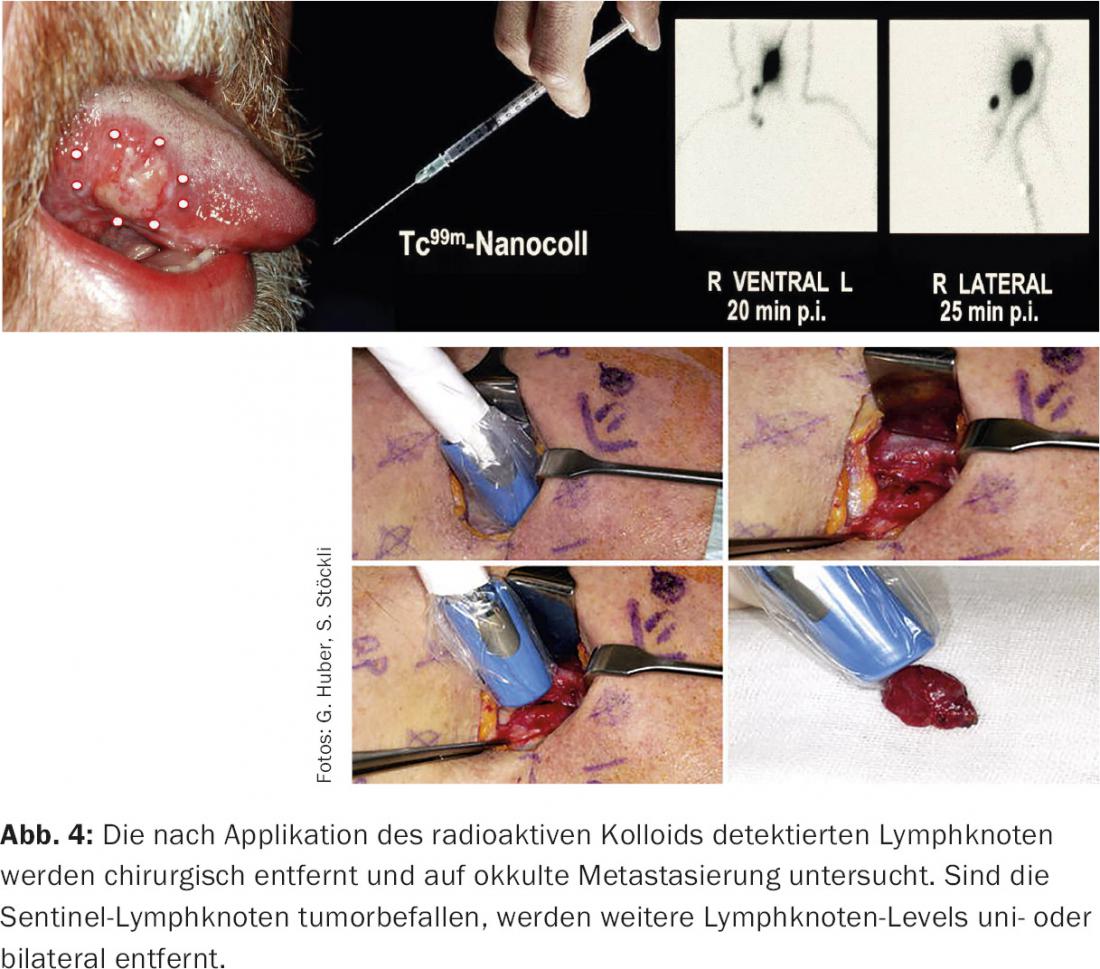
If there is no clinical or imaging evidence of lymph node involvement, there is still a risk of occult metastases in individual risk levels, depending on the location of the primary tumor. These are then electively surgically cleared out (elective neck dissection). Exceptions are situations in which patients are at high risk of anesthesia or when the primary tumor is extremely small (<1 cm): In these cases, “watchful waiting” is justifiable. As a compromise, sentinel lymph node biopsy is an option, especially for oral cavity carcinomas, in which the lymph nodes with the highest risk of metastatic involvement are identified and removed (Fig. 4) . If the sentinel lymph nodes are negative, the risk of other lymph nodes being affected is very small (<5%) [7]. If sentinel lymph nodes are positive, a classic neck dissection is performed.
Recent developments: Lasers and robots
Since the 1980s, CO2 laser has also been used for excision. However, in the oral cavity and pharyngeal region, this offers an advantage over resection with the monopolar knife or scalpel/scissors only in isolated cases. The laser is mainly used for tumors of the larynx or for areas that are difficult to access (e.g. at the base of the tongue). The disadvantage is the obstacle-free straight line that the laser beam needs in order to be effective, although fiber optic cables have also been available for the last ten years, enabling “around the corner” applications.
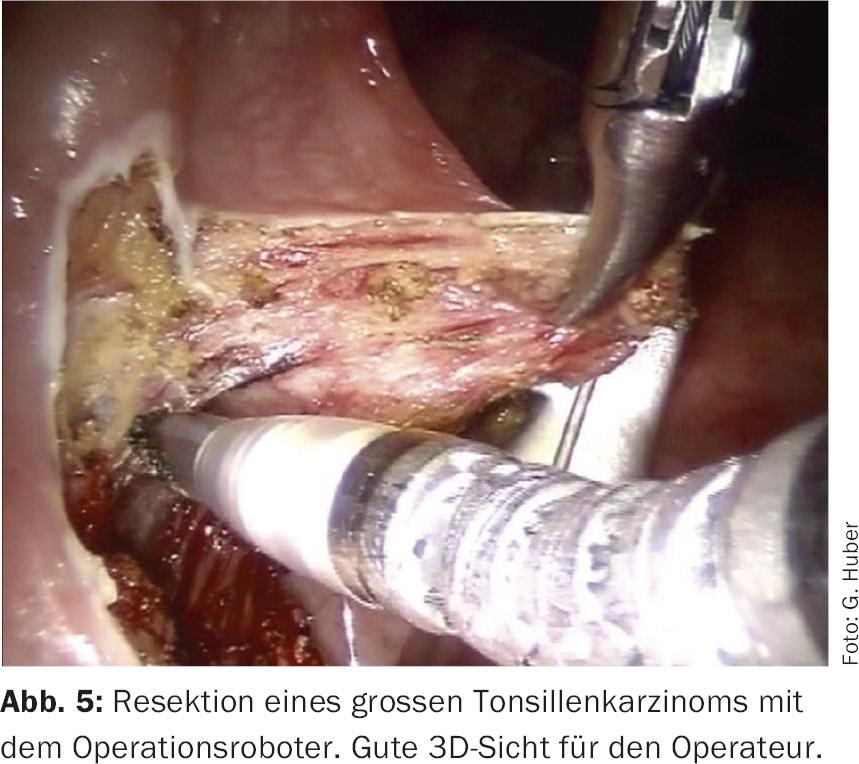
The DaVinci surgical robot has been used successfully in visceral and urological surgery since the 1990s. Since 2005, its application has been extended to the head and neck region [8]. So-called TORS (Trans Oral Robotic Surgery) allows transoral access to sites that previously could only be reached via a mutilating approach (e.g. splitting the mandible). Tumors can thus be removed under optimal 3D visibility (Fig. 5) . These systems are constantly being further developed, and a “single port system” will soon be available for the DaVinci robot (Fig. 6) . DaVinci is also receiving competition for the first time with the Medrobotics system.

Literature:
- Ang KK, et al: Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363(1): 24-35.
- Broglie MA, et al: Impact of high-risk HPV on outcome in oropharyngeal cancer patients treated with primary surgery (submitted).
- Shah KS, et al: Tumour seeding after fine-needle aspiration and core biopsy of the head and neck – a systematic review. Br J Oral Maxillofac Surg 2016 Apr; 54(3): 260-265.
- uicc International Union Against Cancer: TNM Classification of Malignant Tumours,7th Edition, Wiley-Blackwell.
- Strobel K, et al: Head and neck squamous cell carcinoma (HNSCC) – detection of synchronous primaries with (18)F-FDG-PET/CT. Eur J Nucl Med Mol Imaging 2009; 36(6): 919-927.
- Studer G, et al: IMRT in oral cavity cancer. Radiat Oncol 2007; 2: 16.
- Stoeckli SJ: Sentinel node biopsy for oral and oropharyngeal squamous cell carcinoma of the head and neck. Laryngoscope 2007; 117(9): 1539-1551.
- O’Malley BW, et al: Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope 2006; 116(8): 1465-1472.
InFo ONCOLOGY & HEMATOLOGY 2016; 4(6): 18-21.