The third leading cause of cardiovascular disease death is acute pulmonary embolism. The determination of D-dimers is highly sensitive in the exclusion of acute pulmonary embolism. CT angiography is nowadays used to make the diagnosis. After risk stratification, the choice of treatment strategy is made. High-risk patients require aggressive therapy, while low-risk patients may be able to be treated as outpatients. The duration of anticoagulation depends on the presence of provoking factors, bleeding risk, and any evidence of persistently activated coagulation after discontinuation of anticoagulation. Of the new anticoagulants, rivaroxaban is approved in Switzerland as a relapse prophylaxis.
Pulmonary embolism is the third leading cause of death among cardiovascular diseases after myocardial infarction and cerebrovascular insult. In registry studies, mortality at 90 days is found to be between 8.6-17% [1, 2]. Risk factors for high mortality include age >70 years, tumor disease, heart failure, chronic obstructive pulmonary disease (COPD), arterial hypertension, tachypnea, and right ventricular hypokinesia on echocardiography.
Chronic thromboembolic pulmonary hypertension is found in 2-4% of patients after acute pulmonary embolism. This condition is defined as mean pulmonary arterial pressure above 25 mmHg persisting for 6 months after diagnosis of acute pulmonary embolism [3].
Diagnosis
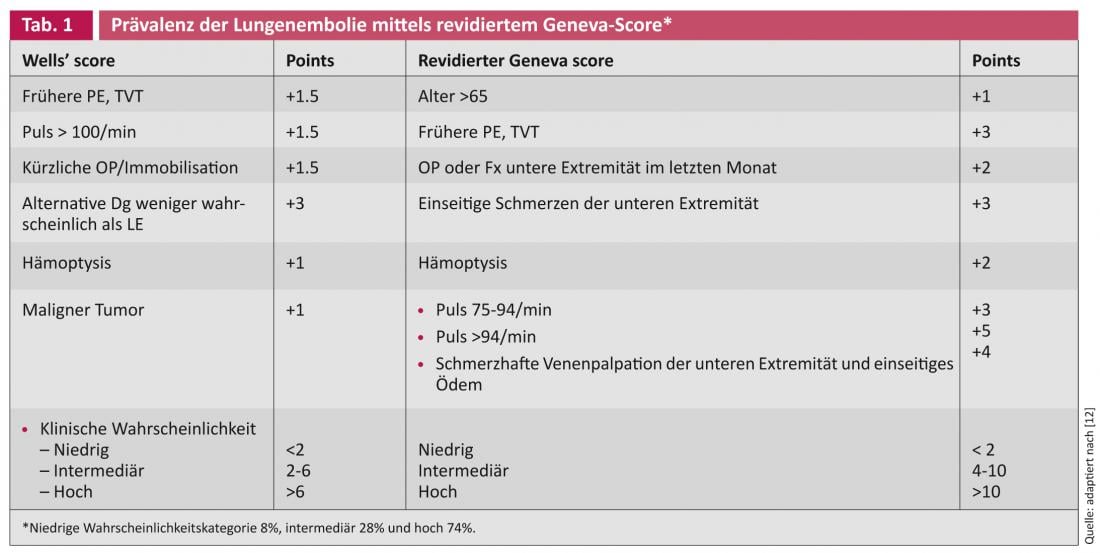
Patients with suspected acute pulmonary embolism should be classified into high (60-80%), intermediate (20-30%), or low (5-10%) levels of suspicion based on their clinical situation. If the probability is low, a normative D-dimer test is sufficient to exclude it. Intermediate and high probability patients require anticoagulation until results are obtained. The history, symptoms, oxygen saturation and ECG are needed to estimate the probability. It can be done empirically or by means of point scores – the Wells Score and the revised Geneva Score are well known [4].
D-Dimer
D-dimer is a degradation product of cross-linked fibrin and is elevated in acute venous thromboembolic events. Analyzed by quantitative ELISA or automated turbidimetric assays, D-dimer determination is highly sensitive (>95%) in ruling out acute pulmonary embolism. In patients with low clinical probability and negative D-dimers, the 3-month risk of a thromboembolic event is very low at approximately 0.1%. However, the specificity of D-dimers is not very high, this concerns mainly patients with high clinical probability, patients assigned for other reasons, >65-year-olds and pregnant women. Age-adjusted D-dimer cutoff values could increase the validity, but prospective validations are still needed.
For example, an international study showed better discrimination by D-dimers in patients over 50 years of age and low clinical probability at a cutoff of (patient age x10) µg/l versus fixed 500 µg/l [5].
CT angiography
Multidetector CT angiography is more sensitive than single-detector CT angiography and has largely replaced ventilation and perfusion lung scintigraphy. Radiation risk should be considered, especially in pregnant women; in these patients, the advantages of CT angiography over pulmonary scintigraphy remain controversial.
Conventional angiography
Conventional pulmonary angiography remains the gold standard for diagnosing pulmonary embolism. Because of its invasiveness, this method should be used only in patients in whom a high-grade suspicion could not be confirmed or in whom endovascular therapy is planned.
Treatment
In patients with acute pulmonary embolism, prognosis assessment should be performed; using the Pulmonary Embolism Severity Index or its simplified version, this can be done based on the clinic [6].
For the therapy of acute pulmonary embolism, the following points should be observed:
– High-risk patients:
- approx. 5% of all symptomatic patients
- Mortality in the acute phase of 15%.
- Should be treated aggressively by thrombolysis, surgically, or catheterization [7]
– Low-risk patients:
- Majority of all patients
- Mortality in the acute phase of about 1%.
- May be treated as a short inpatient or even as an outpatient
– Intermediary risk:
- Affects approximately 30% of symptomatic patients
- Inpatient treatment
- Unclear benefit regarding thrombolysis
Echocardiography, measurements of troponin or pro-brain natriuretic peptide (pro-BNP) may refine risk stratification, but cost-effectiveness is not yet clear.
A multicenter study in Switzerland, France, Belgium, and the United States showed no significant differences in recurrence and bleeding rates compared with inpatient therapy in patients with acute symptomatic pulmonary embolism, low mortality risk (pulmonary embolism severity index risk class I or II), and ambulatory treatment with enoxaparin subcutaneously for at least 5 days followed by vitamin K antagonists [8].

In the acute phase, unfractionated heparin, low-molecular-weight heparins (NMH), fondaparinux, and more recently rivaroxaban are available.
Heparins act by binding to the natural anticoagulant antithrombin, resulting in a massively accelerated inactivation of thrombin by antithrombin. Due to the large individual differences in the binding of heparin to plasma proteins, the dosage must be monitored and adjusted by means of coagulation tests. This is feasible by means of aPTT, thrombin time, anti-FXa activity.
The main advantage of NMH to be administered subcutaneously is the fixed weight-adapted dosage, in most cases without the need for monitoring. The mechanism of action is the same as for unfractionated heparin, but with a stronger effect also on FXa. The clinical equivalence of unfractionated heparin and NMH was demonstrated in one study [9]. Fondaparinux, a synthetic pentasaccharide, at a dosage of 7.5 mg/d is equivalent to unfractionated heparin and NMH in patients with pulmonary embolism.
NMH and fondaparinux are predominantly excreted by the kidney, so caution should be exercised in renal insufficiency with a clearance <30 ml/min. In such a situation, dose reduction, longer intervals, measurement of anti-FXa activity, or use of unfractionated heparin are available alternatives. Patients with malignant tumor disease should be treated with NMH for at least 3 months. When switching to a vitamin K antagonist, overlap with NMH or unfractionated heparin should last at least 5 days and be discontinued after an INR of 2.0 is achieved.
All anticoagulants can cause bleeding. Vitamin K antagonist-associated major bleeding increases with age. Today, clinical scores are validated to estimate the risk of bleeding (RIETE score, HEMORR2HAGES score).
When choosing the duration of anticoagulation, the risk of thromboembolic recurrence should be weighed against the risk of bleeding while on anticoagulation. The 8th ACCP consensus guidelines generally recommend therapy for at least 3 months. This is especially true for patients with provoked events in whom the provoking factor has been eliminated in the interim. In patients with idiopathic events, prolonged therapy may be discussed if the benefit-risk balance is positive. In malignant tumor disease, anticoagulation should be continued until the tumor is controlled or cured. In recent years, an individualized approach has been sought. For example, a study with measurement of D-dimers 4 weeks after discontinuation of anticoagulation showed that risk stratification is possible; low risk with normotensive D-dimers, no further therapy necessary or high risk (15% recurrences after 18 months) with elevated D-dimers with a recommendation to continue anticoagulation for another 12 months [10].
Several new anticoagulants are in development or already approved. These direct, antithrombin-independent inhibitors of FXa (rivaroxaban, apixaban) or thrombin (dabigatran) can replace heparins and vitamin K antagonists in many patients. These drugs are administered in a fixed dosage and do not normally require coagulation monitoring. The Einstein PE trial demonstrated that rivaroxaban at the standard dose (2×15 mg for 3 weeks followed by 20 mg 1x/d) was noninferior to enoxaparin followed by vitamin K antagonists for 3, 6, or 12 months. Significantly less severe bleeding occurred at the same recurrence rate [11]. However, only rivaroxaban has been approved in Switzerland to date, and this only for the recurrent prophylaxis of pulmonary embolism.
Conclusion for practice
- If acute pulmonary embolism is suspected, further workup is performed based on a clinical probability assessment.
- D-dimers have high sensitivity and negative predictive value and can exclude acute pulmonary embolism at low clinical probability.
- For treatment, patients are divided into risk groups; high-risk patients need aggressive treatment quickly, and low-risk patients may be able to be treated as outpatients.
- Treatment is with heparins (unfractionated or NMH) followed by vitamin K antagonists; none of the new substances is currently approved in Switzerland for acute treatment of pulmonary embolism, but recurrence prophylaxis can be given with rivaroxaban.
Thomas Lehmann, MD
Prof. Dr. med. Wolfgang Korte
Literature:
- Goldhaber SZ, et al: Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999;353:1386-1389.
- Laporte S, et al: Clinical predictors for fatal pulmonary embolism in 15 520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation 2008;117:1711–1716.
- Piazza G, Goldhaber SZ: Chronic thromboembolic pulmonary hypertension. N Engl J Med 2011;364:351-360.
- Ceriani E, et al: Clinical prediction rules for pulmonary embolism: a systematic review and meta-analysis. J Thromb Haemost 2010;8:957-970.
- Douma RA, et al: Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: a retrospective analysis of three large cohorts. BMJ 2010;340:c1475.
- Torbicki A, et al: Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J 2008;29:2276-2315.
- Kucher N, et al: Massive pulmonary embolism. Circulation 2006;113:577-582.
- Aujesky D, et al: Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. The Lancet 2011;378:41-48.
- Simonneau G, et al: A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. N Engl J Med 1997;337:663-669.
- Palareti G, et al: D-dimer testing to determine the duration of anticoagulant therapy. N Engl J Med 2006;355:1780-1789.
- Büller HR, et al: Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287-97.
- Le Gal G, et al: Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.