Loss of renal function can lead to accumulation of uremic toxins or life-threatening changes in electrolyte and volume balance. This negative development can be countered with various dialysis procedures.
Acute or chronic kidney failure can be caused by various factors. If loss of renal function results in accumulation of uremic toxins or life-threatening electrolyte and volume shifts, dialysis is used as a renal replacement procedure. Hemodialysis (HD) is based on the physical principle of diffusion, which removes small-molecule molecules from the blood along their concentration gradient across a semipermeable membrane. Hemofiltration (HF), on the other hand, is based on convection, which eliminates the plasma water through the transmembrane pressure difference (ultrafiltrate) and removes the molecules dissolved in it (“solvent drag”). Hemodiafiltration (HDF) combines the principles of diffusion and convection.
Dialysis for acute renal failure
Acute renal failure (ANV) is one of the most common complications in hospitalized patients, occurring in up to 20% of all cases. Critically ill patients in intensive care units with sepsis or major surgery, patients with preexisting renal insufficiency, and those with chronic diseases of other organs (e.g., heart failure or diabetes mellitus) are particularly at risk [1]. The occurrence of ANV is an independent prognostic factor; a creatinine increase of 26.5 µmol/l is already associated with a doubling of mortality.
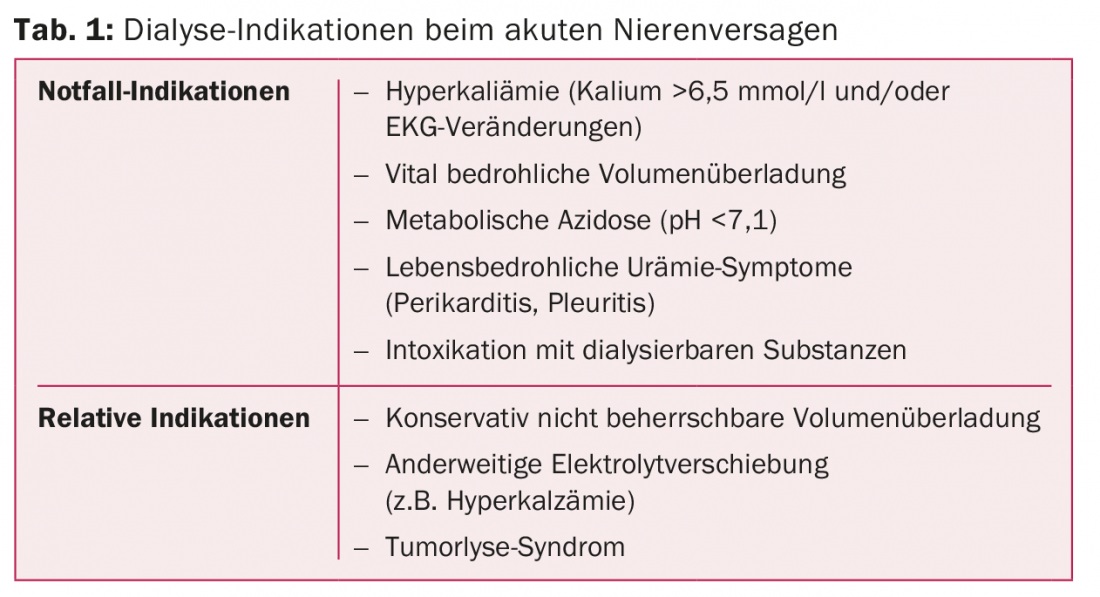
The indications for emergency dialysis are life-threatening electrolyte shifts, especially hyperkalemia (potassium >6.5 mmol/l and/or ECG changes), volume overload (pulmonary edema), severe metabolic acidosis (pH <7,1), complications of uremia (pericarditis), or intoxications with dialyzable substances (e.g., salicylates, alcohols). Relative indications for dialysis are refractory electrolyte shifts and volume overload or severe hyperuricemia, as occurs in tumor lysis syndrome (Table 1).
Many studies postulate that in patients with ANV without emergency indications, early initiation of dialysis immediately after diagnosis does not provide a significant survival benefit [2,3]. Few data suggest a benefit of early dialysis use [4]. However, delayed onset may offer the possibility of spontaneous renal recovery without the use of renal replacement procedures [2,3]. Because of the controversial data, the KDIGO (“Kidney Disease: Improving Global Outcomes”) guidelines do not recommend a specific time point or specific creatinine or urea threshold for dialysis initiation [5].
The average duration of a dialysis procedure in an ANV is 12-13 days [6]. Based on this, a double-lumen, non-tunnelled central catheter, preferably in the right jugular vein, is recommended as a temporary access [5]. In contrast to outpatient dialysis for chronic patients, continuous (over 24 hours) dialysis procedures are available for ANV in addition to intermittent treatment (two to four hours every 1-2 days). The advantages of continuous dialysis are the slower and thus more circulatory-friendly withdrawal of fluid and dialyzable molecules. The advantages of intermittent dialysis are rapid removal of toxins in cases of intoxication and limited treatment time, which allows better coordination with diagnostic or interventional therapies. Special attention should be paid to adjust drug dosages to each modality. This is crucial for antibiotics, for example.
Various studies and meta-analyses show no significant difference in mortality or length of hospital stay in patients on continuous versus intermittent dialysis. Interestingly, however, a more rapid renal recovery is repeatedly postulated after the use of continuous dialysis, although recent studies do not confirm this [7]. Therefore, the KDIGO recommendations list intermittent and continuous procedures as equivalent. Continuous dialysis is recommended only in hemodynamically unstable patients and in those with increased intracranial pressure or cerebral edema [5].
Estimating the adequate dialysis dose is complex in the context of an ANV because patients’ metabolic ratios change daily. The optimal dialysis dose of intermittent hemodialysis is expressed using urea clearance (Kt/V = urea clearance × time per urea distribution volume); the KDIGO recommends a Kt/V of 3.9 per week for ANV [5]. The treatment intensity of continuous dialysis, on the other hand, is indicated by the total effluent rate (ultrafiltrate rate and dialysate outflow volume). Based on a number of studies, a total outflow rate of 20-25 ml/kg/h is recommended [5,6]. More intensive therapy does not result in survival benefit or more rapid renal recovery in either intermittent or continuous dialysis [6] and is even harmful in septic patients. It is important to prescribe a higher dose, as the effective dose administered may differ by up to 20% from the target dose due to interruptions in therapy caused by, for example, access problems or hemodynamic instability.
Thus, in the context of an ANV, the clinical context, underlying disease, and presence of other organ dysfunction is critical to prescribing the right dialysis for each patient based on individual circumstances.
Dialysis for chronic renal failure
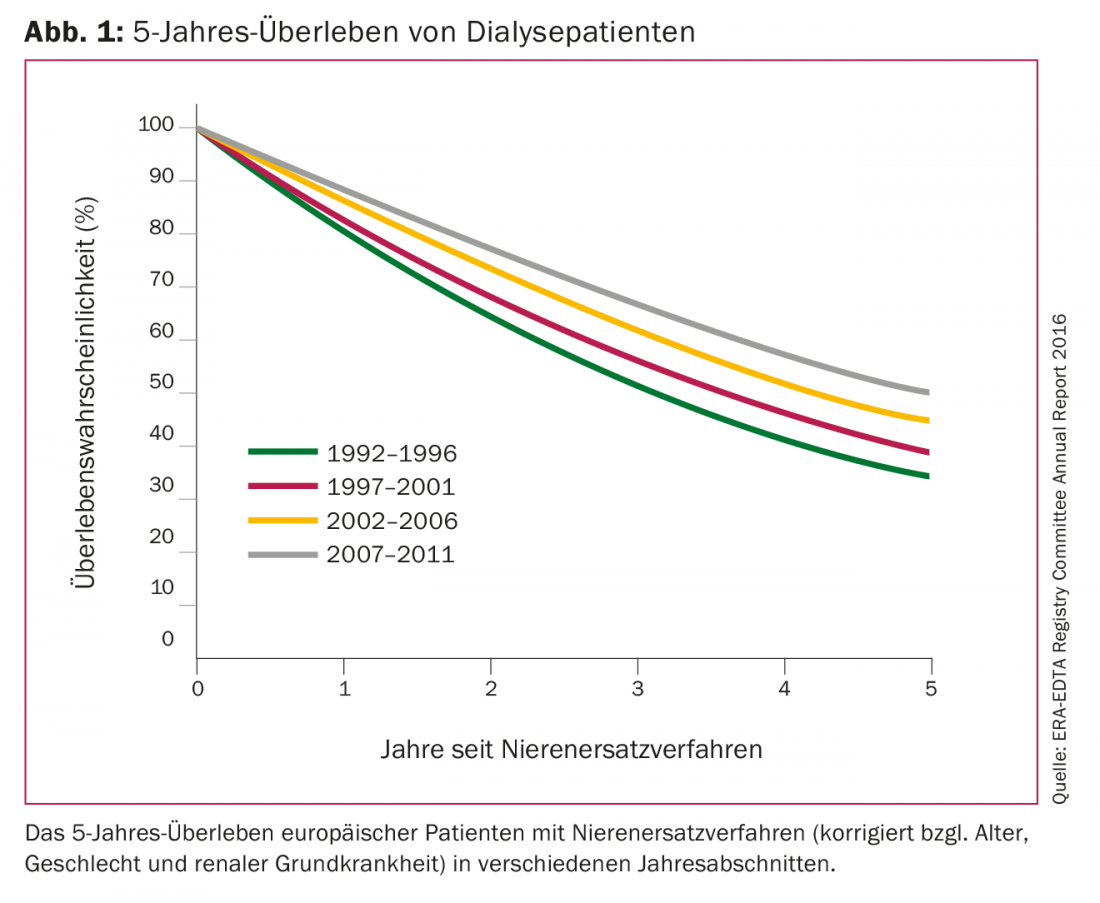
The mortality of chronic dialysis patients is increased ten- to twenty-fold compared to the age-matched general population. Cardiovascular disease is considered the predominant cause of death. According to the United States Renal Data System, the 1-year mortality rate for U.S. dialysis patients in 2016 was just under 20%, comparable to rates for malignant tumors. Among European dialysis patients, 5-year survival was only 35% 25 years ago; by 2011, it had risen to just under 50% (Fig. 1). Various new discoveries in the field of optimal dose, frequency and procedures of dialysis as well as the biocompatibility of filter membranes and the development of recombinant erythropoietin have contributed to this significant improvement in patient survival.
In 1980, the first randomized controlled trial of nephrology first investigated the optimal dialysis dose and introduced a Kt/V >1.0 per treatment as the minimum dose to aim for [8]. More recent data confirm a significant mortality increase at a Kt/V <1.2 per dialysis [9] and reinforce the association of better patient survival with higher dialysis dose. However, the landmark HEMO trial showed no further survival benefit with a Kt/V >1.3 per treatment, although some shortcomings were pointed out regarding the methodology used in the trial (young study population, carry-over effect) [10]. In summary, the recommended target dialysis dose today is Kt/V >1.2 per dialysis [11].
Standard dialysis therapy worldwide comprises three treatments per week, each lasting three to five hours. More intensive therapy is defined as ≥5 dialysis sessions per week that are either short (<3 hours), regular (3-5 hours), or long (>5 hours) [11]. The Frequent Hemodialysis Network (FHN) published three randomized trials, each comparing intensive treatments (6×/week of 1.5-3 hours; 6×/week of >6 hours/night; and 5-6×/week of >6 hours/night) with standard frequency. All showed significant improvement in quality of life, reduction in left ventricular hypertrophy, and better control of arterial hypertension and hyperphosphatemia in the cohorts with more intensive treatment. In contrast, these patients had more frequent vascular access interventions. Interestingly, long-term follow-up demonstrates a survival benefit only in cohorts with daily dialysis of regular duration. However, methodological factors influencing the studies are to be criticized (small number of patients, low statistical power). More intensive nocturnal dialysis accelerates the loss of residual renal function. This is of pronounced relevance in dialysis patients, not only for the elimination of small and medium-sized molecules, but also in terms of prognosis [12]. Thus, residual diuresis is a critical parameter for optimal dialysis therapy. Based on this, the concept of incremental dialysis has developed in recent years. If residual function is still preserved, one to two treatments per week are started initially and the standard dose of three times per week is not introduced until urea clearance <3 ml/min/1.73 m2 or residual diuresis <600 ml/day [12]. Patients with few comorbidities, minor electrolyte disturbances, and compensated volume balance are most likely to benefit from this individualized dialysis prescription.
The optimal time to initiate renal replacement has been when there is a decrease in glomerular filtration rate (GFR) <15 ml/min/1.73 m2 and/or signs of uremia. However, the IDEAL study shows no survival benefit of early dialysis initiation (GFR 10-14 ml/min/1.73m2 versus 5-7 ml/min/1.73 m2) [13], so waiting until GFR of 7 ml/min/1.73 m2 and/or uremic symptoms can be considered. Special attention is needed for elderly patients, who represent an increasing proportion of the dialysis population. Quality of life does not differ with early or late initiation of dialysis [13], but initiation of dialysis in the elderly leads to a marked increase in mortality and a rapid decline in independence [14].
Furthermore, hemodiafiltration (online-HDF) has been increasingly investigated in recent years in the hope of improving dialysis patient survival with more effective elimination of medium-sized molecules. A pooled analysis of four prospective randomized trials demonstrates a significant reduction in both all-cause mortality and cardiovascular death in dialysis patients on HDF versus high-flux HD according to [15]. However, this effect is only noticeable from a convection volume of at least 23 liters per treatment and is significantly evident after standardization to the entire body surface. However, to achieve the high convection volumes, a corresponding blood flow (>300 ml/min) and a longer dialysis time are required, which presupposes good vascular access.
Furthermore, the development of recombinant erythropoietin in the late 1980s was a groundbreaking advance in the treatment of dialysis patients. Previously, dialysis patients regularly had low hemoglobin levels of 60-80 g/l, which required repeated blood transfusions and were associated with corresponding morbidity risks. With the use of recombinant erythropoietin, improvement in anemia-associated symptoms and quality of life as well as an evidenced reduction in the need for transfusion was seen within a short time. Four landmark studies investigated the target hemoglobin level and its impact on mortality in patients with chronic renal failure. In summary, increased thromboembolic events and cardiovascular death are seen when hemoglobin normalizes to ≥130 g/l. Thus, the most current guidelines recommend a hemoglobin level of 110-115 g/l as the target in dialysis patients [16].
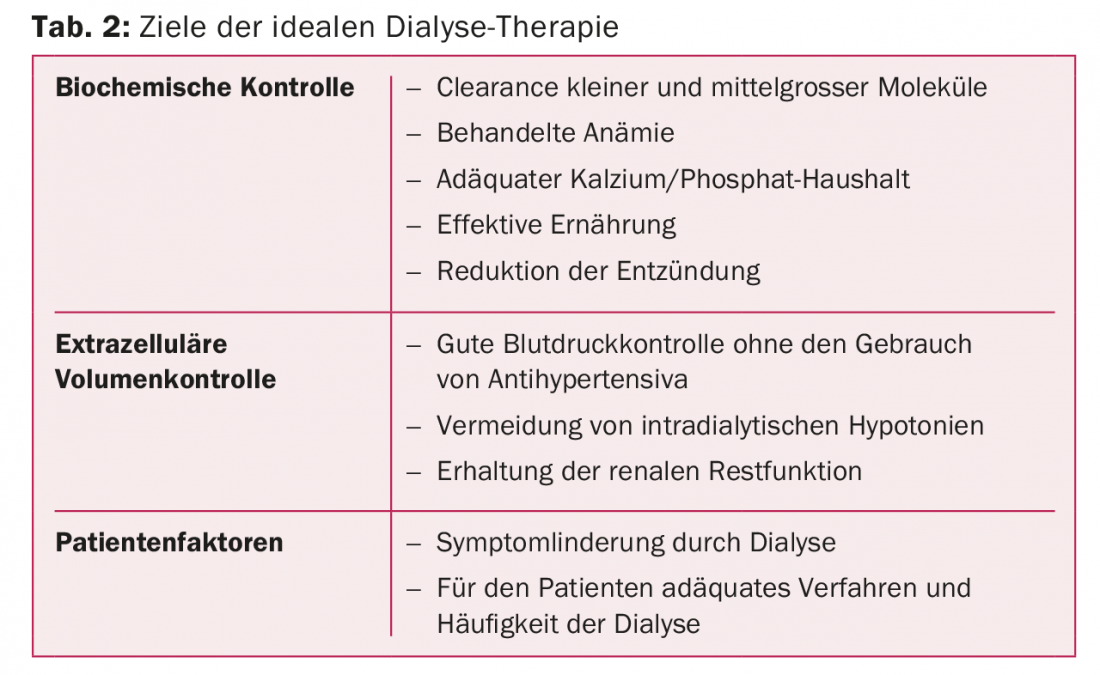
It is therefore crucial that the right dialysis is planned and scheduled individually for each patient in order to improve patient survival without compromising quality of life. The characteristics of ideal dialysis are listed in Table 2.
Take-Home Messages
- In the setting of acute dialysis, there is no survival benefit with intermittent versus continuous dialysis. A continuous procedure is recommended in hemodynamically unstable patients and those with increased intracranial pressure.
- Early dialysis initiation is not associated with better survival in either acute or chronic dialysis therapy.
- Renal residual function is prognostically relevant and allows for an individually designed dialysis prescription.
- The survival benefit of hemodiafiltration is not seen in chronic dialysis patients until a convection volume of >23 liters per session.
Literature:
- Rewa O, Bagshaw SM: Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 2014; 10: 193-207.
- Gaudry S, et al: Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 2016; 375: 122-133.
- Barbar SD, et al: Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 2018; 379: 1431-1442.
- Zarbock A, et al: Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN Randomized Clinical Trial. JAMA 2016; 315(20): 2190-2199.
- Khwaja A: KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120(4): c179-184.
- Palevsky PM, et al: Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008; 359: 7-20.
- Liang KV, et al: Modality of RRT and recovery of kidney function after AKI in patients surviving to hospital discharge. Clin J Am Soc Nephrol 2016; 11(1): 30-38.
- Lowrie EG, et al: Effect of the hemodialysis prescription of patient morbidity: report from the National Cooperative Dialysis Study. N Engl J Med 1981; 305(20): 1176-1181.
- Port FK, et al: DOPPS estimates of patient life years attributable to modifiable hemodialysis practices in the United States. Blood Purif 2004; 22(1): 175-180.
- Eknoyan G, et al: Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 2002; 347: 2010-2019.
- National Kidney Foundation: KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am J Kidney Dis 2015; 66(5): 884-930.
- Obi Y, et al: Incremental hemodialysis, residual kidney function, and mortality risk in incident dialysis patients: a cohort study. Am J Kidney Dis 2016; 68(2): 256-265.
- Cooper BA, et al: A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363: 609-619.
- Kurella Tamura M, et al: Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009; 361(16): 1539-1547.
- Peters SA, et al: Haemodiafiltration and mortality in end-stage kidney disease patients: a pooled individual participant data analysis from four randomized controlled trials. Nephrol Dial Transplant 2016; 31(6): 978-984.
- Kidney Disease Improving Global Outcomes (KDIGO): KDIGO clinical practice guidelines for anemia in chronic kidney disease. Kidney International Supplements 2012; 2(4).
A complete list of all sources used is available upon request.
CARDIOVASC 2018; 17(6): 8-12











