In bright sunny early spring weather, it was hard to stay in the lightless lecture halls at the 25th Physicians’ Continuing Education Course in Clinical Oncology in St. Gallen, but it was worth it. PD Dr. med. Markus Jörger, Oncology and Hematology, St. Gallen, provided comprehensive information on drug interactions in oncological therapies in his presentation.
The definition of drug interactions (AMI) is as follows: “A measurable change in the specific drug effect (in magnitude or duration) due to the prior or concurrent administration of another drug.” In practice, however, AMI can be caused not only by drugs but also by foods, herbal substances, noxious agents such as alcohol and smoking, and other chemical substances. The risk for AMI increases with the number of medications administered, use of drugs with low therapeutic breadth, increasing impairment of organ function (liver, kidney), and comorbidities. In addition, people with special sensitivities (obesity, cachexia, old age) are more affected by AMI.
Pharmaceutics, -kinetics and -dynamics
Basically, AMI can be divided into three forms:
- Pharmaceutical interactions are due to the chemical properties of the active ingredients. For example, the pH dependence of the gastric absorption of a drug is clinically significant.
- In pharmacokinetic interactions, one drug affects the absorption, distribution, metabolism, or elimination (ADME) of another drug. Changes in plasma concentrations affect the activity or toxicity of another agent, so that a dose reduction or increase may be necessary. Hepatic CYP-P450 enzymes and renal elimination of drugs are important.
- In pharmacodynamic interactions, one drug alters the efficacy of a second drug without decreasing or increasing its plasma concentration. These interactions can also be used therapeutically: Typical examples are the combination of different antihypertensives, the administration of antagonists in cases of intoxication or the combination of cytostatics in oncology.
Caution with food
AMI occur frequently: According to estimates, 7% of all adverse drug reactions (ADRs) in hospitalized patients and 30% of all fatal ADRs are due to AMI. The more medications a patient takes, the higher the risk.
In principle, most AMI are avoidable if one is aware of the risk and the particular risk situations (Tab. 1). High-risk drugs include not only the well-known substance groups (oncologics, NSAIDs, anticoagulants, etc.), but also herbal preparations such as echinacea and St. John’s wort. The latter is a strong CYP inducer and may thereby reduce the efficacy of other drugs. The two most significant effects of St. John’s wort are on the effects of ciclosporin (organ transplant rejection crises) and contraceptives (intermenstrual bleeding and unwanted pregnancies). Soy contains flavonoids with proestrogenic effects, so patients with breast cancer and tamoxifen treatment should be cautious when taking soy products.
Drug-food interactions can also play an important role, especially with oral oncologics. A high-fat meal increases the absorption of tyrosine kinase inhibitors (TKIs) and thus the risk for side effects. Fasting is therefore recommended for most TKIs, although there are exceptions such as imatinib or dasatinib. “To avoid AMI as much as possible, you have to inform patients when to take their medications,” Dr. Jörger emphasized. “In St. Gallen, the nurses, who also distribute the medications, have corresponding fact sheets so that they can pass on the information in detail to the patients.” Fruit juices (apple juice, grapefruit juice, orange juice, etc.) can also be problematic: They slow excretion, increasing the risk for AMI and complications such as QT prolongation, arrhythmias, or myelosuppression.
In the second part of his presentation, Dr. Jörger presented various cases of AMI.

Capezitabine and Marcoumar
A 66-year-old patient with metastatic breast carcinoma had been receiving Marcoumar since January 2007 because of bilateral pulmonary emboli. From January 2008, therapy with Capezitabine (Xeloda®) was initiated. In November, the patient developed a chronic subdural hematoma. The reason for this was quickly found: Capezitabine is a strong CYP2C9 inhibitor and thus prevents the degradation of Marcoumar. Dr. Jörger said patients on Xeloda® treatment should be switched from Marcoumar to a low-molecular-weight heparin (LMWH) to avoid bleeding complications.
Erlotinib and simvastatin
A 75-year-old female patient with adenocarcinoma of the lung was treated with erlotinib (Tarceva®) as second-line therapy. After six weeks, emergency admission was made with muscle pain and asthenia, and a diagnosis of active rhabodmyolysis was made. Because of corresponding comorbidities, the patient had taken aspirin, atenolol, amlodipine, and simvastatin for years without complications. Inhibition of CYP3A4 by erlotinib had increased plasma concentrations of simvastatin, resulting in rhabdomyolysis. Stopping simvastatin following the recovery period allowed for trouble-free continued treatment with erlotinib.
The oral EGFR TKI erlotinib interacts with a whole range of substances. Due to CYP3A4 induction, there is a risk of reduced efficacy when combined with agents such as phenytoin, St. John’s wort, dexamethasone, etc. This problem also exists in smokers, who have significantly lower plasma levels than nonsmokers at the standard dose of 150 mg/d. On the other hand, the combination of erlotinib and azoles, grapefruit juice, macrolides, Ca antagonists, nelfinavir, etc., carries a risk of increased toxicity. Concomitant use of erlotinib and proton pump inhibitors (PPIs) should be avoided if possible or should be timed separately (erlotinib in the morning, PPI in the evening).
Sunitinib and carbamazepine
In a 76-year-old patient with metastatic renal cell carcinoma treated with carbamazepine and phenytonin, therapy with sunitinib (Sutent®) was under discussion. Sunitinib is a CYP3A4 substrate, and carbamazepine and phenytoin are potent CYP3A4 inducers. In this situation, switching to a non-enzyme-inducing antiepileptic drug (non-EIAED) by the neurologist is recommended (Table 2) . If the combination of sunitinib and an EIAED is unavoidable, a gradual dose increase of sunitinib should at least be considered if well tolerated to avoid loss of efficacy.
Capecitabine and diclofenac
A 62-year-old patient with rectal cancer received adjuvant chemotherapy with capecitabine (Xeloda®). After five months, he was referred on an emergency basis with neutrogenic fever, diarrhea, rash, and erythema on the palms and soles of his feet. A diagnosis of hand-foot syndrome and subacute renal dysfunction was made. The patient had been taking high doses of diclofenac for acute lumbalgia.
AMI with methotrexate
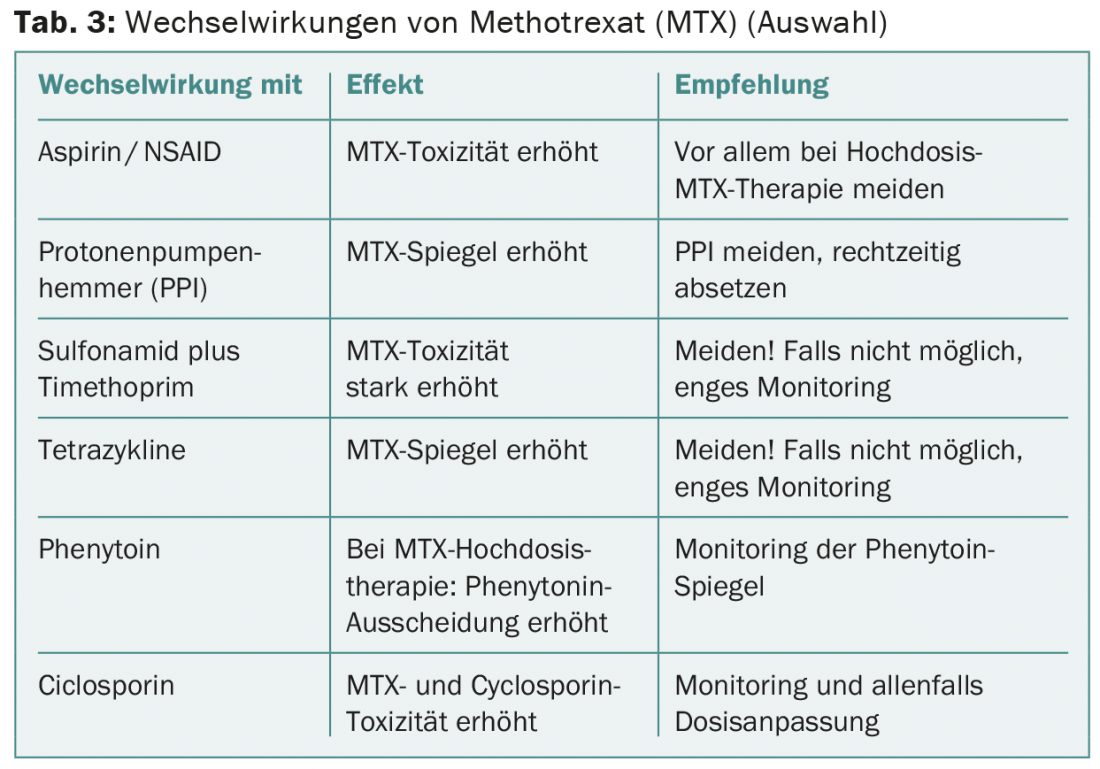
Methotrexate (MTX) interacts with many other drugs (Table 3) . A 65-year-old patient with laryngeal carcinoma was treated with MTX 60 mg i.v. after local recurrence. One week later, he suffered from severe stomatitis, ulcerative exanthema, and severe neutropenia. This was accompanied by pneumonia, from which the patient ultimately died. He had been taking mefenazide intermittently, which in this case likely resulted in subacute renal dysfunction and severely delayed renal elimination of MTX.
AMI with imatinib
A 75-year-old patient with chronic myeloid leukemia (CML) started imatinib therapy (Gleevec®). A year later, he was referred on an emergency basis with upper abdominal pain, jaundice, and severely elevated liver enzymes. A diagnosis of subacute lobular hepatitis was made, most likely toxic-medicinal. Two months earlier, the patient had started taking a ginseng preparation. However, ginseng is an inhibitor of the CYP3A4 system and also inhibits the degradation of imatinib, which had resulted in increased plasma imatinib levels and severe toxicity in this patient. After discontinuation of the drugs, liver enzymes normalized within three weeks, and imatinib was readily reintroduced.
The combination of imatinib and simvastatin is also problematic. Therefore, before starting imatinib therapy, it is recommended to switch from simvastatin to pravastatin.
Furthermore, caution should be exercised when paracetamol and imatinib are administered concomitantly, as liver toxicity may also occur with this combination. The corresponding maximum daily dose of paracetamol is around 1300 mg and should not be exceeded if possible.
Conclusion for practice
- AMI are common and clinically significant.
- Many AMI can be avoided.
- Avoid polypharmacy as far as possible: Look carefully at the list of medicines and assess whether something can be omitted or changed.
- Knowledge of specific comedic medications and supplements is important.
- Medication compliance support.
- Prompt patients to tell if they are swallowing additional products (OTC medications, herbal or alternative remedies).
Source: 25th Physician Continuing Education Course Clinical Oncology, St. Gallen, February 19-21, 2015.
InFo ONCOLOGY & HEMATOLOGY 2015; 3(3-4), 30-32.