Given the high incidence of stroke events and subsequent cognitive impairment, there is a great need for new therapeutic options that can be used in early post-stroke phases. A randomized multicenter trial in an open-label design investigated the effects of a standardized ginkgo preparation on cognitive function in patients after a recent mild or moderate ischemic stroke.
Several large population-based studies found that cognitive impairment manifested more rapidly in stroke victims compared with the normal population during long-term follow-up [1,2]. The underlying mechanisms, extent, and possible predictors are not fully understood to date. Other than brain stimulation techniques and physical activity, there are few options to date to treat cognitive decline in this patient population [3]. The ginkgo special extract EGb 761® has already repeatedly demonstrated in humans blood circulation-promoting effects in the brain and an improvement in the blood’s flow properties [4]. In a meta-analysis published in 2015, Ginkgo biloba led to stabilization of cognitive performance or slowing of performance decline in individuals with cognitive impairment or dementia [5]. In a small randomized-controlled six-month trial of Ginkgo biloba in patients with vascular cognitive impairment ( VCI**) published in 2017, a positive effect could be observed with respect to the Clinical Global Impression scale (CGI) [6]. Moreover, there are findings from in vivo ischemia studies in which Ginkgo biloba reduced infarct size, neurological deficits, and motor dysfunction. With this in mind, it is natural to investigate the efficacy of EGb 761® for the treatment of cognitive deficits in patients after ischemic stroke [7].
** VCI = any cognitive impairment associated with cerebrovascular injury or ischemic/hemorrhagic stroke.
Ginkgo biloba extract as add-on to standard treatment
For the present study, 201 patients aged ≥50 years who had suffered an ischemic stroke 7-14 days previously and scored ≤20 or less on the NIH (National Institutes of Health) stroke scale were recruited from seven Chinese medical centers [7]. Exclusion criteria included dementia diagnosed before stroke or other serious neurological disease (e.g., Parkinson’s disease, Huntington’s disease, Creutzfeldt-Jakob disease) and mental disorders (e.g., major depression, generalized anxiety disorder). All included patients received standard guideline-based treatment to prevent recurrent stroke, including general supportive care, antiplatelet agents, and measures for acute complications; nootropics were not allowed. 100 patients were randomly assigned to add-on treatment with 240 mg/d EGb 761®, while 101 patients received standard treatment alone, without add-on therapy (=control group) [7]. Various relevant parameters were collected from all study participants during a follow-up period of 24 weeks.
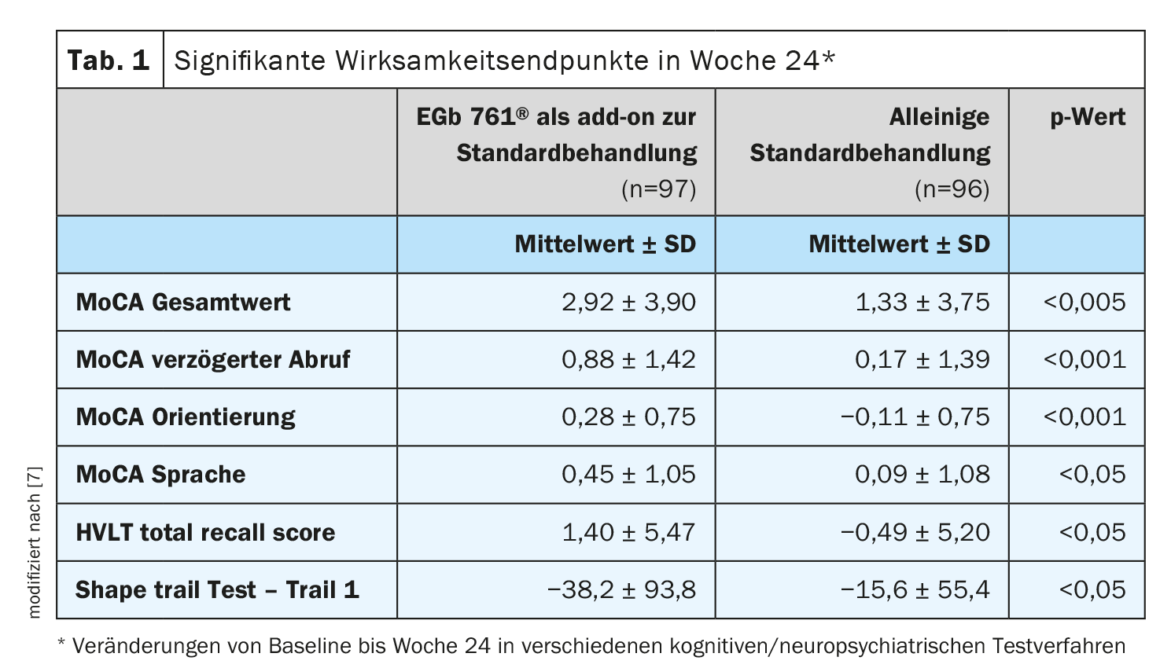
Significant improvements manifested in the EGb 761® group
At week 24, the Ginkgo group showed significant improvements in the Montreal Cognitive Assessment (MoCA) and other test domains compared with the KG (Table 1, Review 1) [7]:
- MoCA total score: mean change since baseline was 2.92 vs. 1.33 (95% CI; 0.51-2.67; p<0.001). This corresponds to a performance 1.59 points better than in the KG. The mean change in the overall MoCA value was thus clearly in favor of the EGb 761® group.
- MoCA subscore delayed retrieval: change of 0.88 vs. 0.17 in the KG (95% CI; 0.31-1.10; p<0.001).
- MoCA subscore orientation: change of 0.28 vs -0.11 in the KG (95% CI; 0.18-0.60; p<0.001).
- MoCA subscore language: change of 0.45 versus 0.09 in the KG (95% CI; 0.06-0.66; p<0.05).
- Hopkins Verbal Learning Test (Total Recall): 1.89 points more improvement than KG (p<0.05)
- Shape Trail Test-Trail 1: 22.6 points better improvement than KG (p<0.05)
Furthermore, in the EGb 761® group, a proportion of 80.2% achieved a great or very great improvement in the Clinical Global Impression of Change scale (CGI-C) compared to baseline. In the control group (KG), this was true for only 20.8% of patients. In contrast, some tests showed no significant differences between the EGb 761® group and the KG at week 24: Shape Trail Test Trail 2, Verbal Fluency Test, Digit Symbol Substitution Test of the Wechsler Adult Intelligence Scale-Revised, Hospital Anxiety and Depression Scale, neuropsychiatric index.
Beneficial risk-benefit profile
The incidence of adverse events was similar in both study arms [7]. It was 11.1% in the EGb 761® group and 9.9% in the KG. Nasopharyngitis was reported most frequently, affecting 3% of each of the two study arms. Most adverse events were mild to moderate, with a total of five serious adverse events reported: one patient each with cerebral infarction and chronic renal failure in the EGb 761® group; in the KG, two cerebral infarctions occurred in one patient, and a lacunar infarction in another. A causal relationship with treatment could not be excluded in the following cases: Skin rash, dizziness (possibly treatment-related), and chronic renal failure (association with treatment unlikely). While there was evidence in several previously published studies that EGb 761® may increase bleeding risk [8,9]In the present study, the results support the assumption that the use of EGb 761® at a dose of 240 mg/d over a period of 24 weeks has a favorable effect on cognitive performance, but without increasing the risk of bleeding. This is consistent with studies showing that taking EGb 761® paralell to anticoagulants/antiplatelets does not increase the risk of bleeding [19,20].
| Conclusion Consistent with previous findings, the results of the present study indicate that EGb 761® at a dose of 240 mg/d for 24 weeks is a valid, well-tolerated treatment option for alleviating cognitive symptoms and improving quality of life in patients after ischemic stroke [7]. EGb 761® was found to result in slight improvements in cognitive performance, mainly reflected in a greater increase in MoCA scores compared to the control group. The Montreal Cognitive Assessment (MoCA) has been shown to be a sensitive measure to map changes in cognitive functions, especially regarding executive deficits, which have prognostic relevance for cognitive impairment after stroke [14,15]. In this study, only patients with mild neurological disorders were included. In general, it can be stated that drug interventions in an early phase of cognitive impairment are more sustainable with regard to the maintenance of corresponding functional domains [16,17]. Ginkgo biloba extract contains several components that have been shown to stimulate blood flow to the brain, reduce oxidative stress, and increase neurotransmitter activity (e.g., acetylcholine), which is relevant to learning and memory functions [18]. In summary, the authors hold that the neuroprotective effects of EGb 761® have a modest but consistent beneficial effect on slowing or stabilizing the progression of cognitive impairment when ginkgo extract is used early enough. |
Literature:
- Zheng F, et al.: Progression of cognitive decline before and after incident stroke. Neurology 2019; 93(1), e20–e28.
- Lo JW, et al.: Long-term cognitive decline after stroke: An individual participant data meta-analysis. Stroke 2022; 53(4): 1318–1327.
- Bordet R, et al.: Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: A consensus report. BMC Med 2017; 15 (1): 107.
- Drug information, www.swissmedicinfo.ch,(last accessed 13.06.2023).
- Tan M-S, et al.: Efficacy and adverse effects of Ginkgo Biloba for cognitive impairment and dementia: a systematic review and meta-analysis. J Alzheimer‘s Dis 2015; 43: 589–603.
- Demarin V, et al.: Efficacy and safety of Ginkgo biloba standardized extract in the treatment of vascular cognitive impairment: a randomized, double-blind, placebo-controlled clinical trial. Neuropsychiatr Dis Treat 2017; 13: 483–490.
- Cui M, et al.: Ginkgo biloba extract EGb 761® improves cognition and overall condition after ischemic stroke: Results from a pilot randomized trial. Front Pharmacol. 2023 Mar 29; 14: 1147860.
- Kudolo GB, Dorsey S, Blodgett J: Effect of the ingestion of Ginkgo biloba extract on platelet aggregation and urinary prostanoid excretion in healthy and Type 2 diabetic subjects. Thrombosis Res 2002; 108(2–3): 151–160.
- Bent S, et al.: Spontaneous bleeding associated with ginkgo biloba: A case report and systematic review of the literature: A case report and systematic review of the literature. J general Intern Med 2005; 20 (7): 657–661.
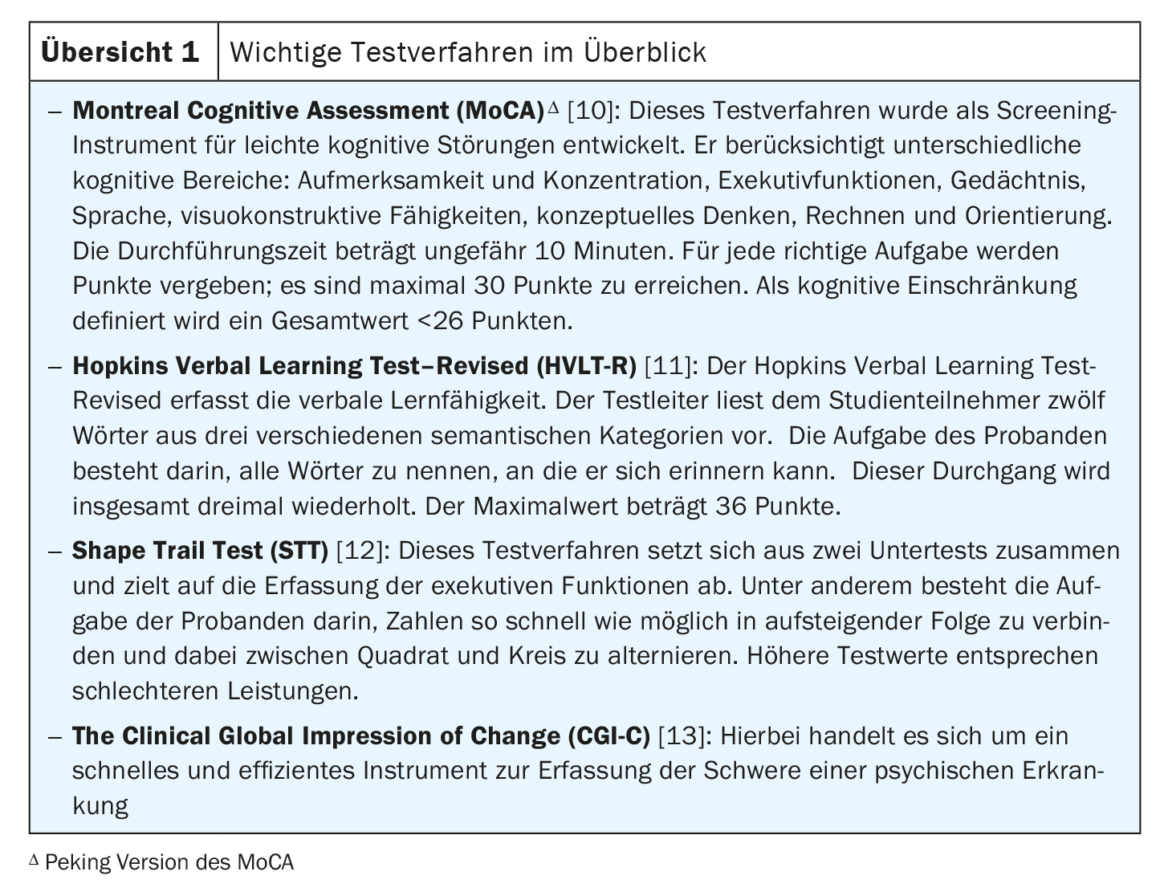
- Julayanont P, Nasreddine ZS: Montreal cognitive assessment (MoCA); in Concept and clinical review bt – cognitive screening instruments: A practical approach. Editor Larner A. J. (Cham: Springer International Publishing), 2017; 139–195.
- Shi J, et al.: The utility of the Hopkins Verbal Learning Test (Chinese version) for screening dementia and mild cognitive impairment in a Chinese population. BMC Neurol 2012; 12: 136.
- Zhao Q, et al.: The Shape Trail Test: Application of a new variant of the Trail Making Test. PLoS ONE 2013; 8(2), e57333.
- Busner J, Targum SD: The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry (Edgmont Pa, Townsh) 2007; 4(7): 28–37.
- Ritter A, Pillai JA: Treatment of vascular cognitive impairment. Curr Treat Options Neurology 2015; 17(8): 367.
- Tan HH, et al.: Decline in changing Montreal Cognitive Assessment (MoCA) scores is associated with post-stroke cognitive decline determined by a formal neuropsychological evaluation. PloS one 2017; 12(3): e0173291.
- Lissek V, Suchan B: Preventing dementia? Interventional approaches in mild cognitive impairment. Neurosci Biobehav Rev 2021: 122: 143–164.
- Marcolini S, et al.: Effects of interventions on cerebral perfusion in the Alzheimer’s disease spectrum: A systematic review. Ageing Res Rev 2022; 79: 101661.
- Ahlemeyer B, Krieglstein J: Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci 2003; 60(9): 1779–1792.
- Băjenaru O, et al.: Effectiveness and Safety Profile of Ginkgo Biloba Standardized Extract (EGb 761®) in Patients with Amnestic Mild Cognitive Impairment. CNS Neurol Disord Drug Targets 2021, Feb 8, (online ahead of print), doi: 10.2174/1871527320666210208125524.
- Hoerr R: Single and Repeated Doses of EGb 761® do not Affect Pharmacokinetics or Pharmacodynamics of Rivaroxaban in Healthy Subjects. Front Pharmacol 2022, 20 April, https://doi.org/10.3389/fphar.2022.868843
HAUSARZT PRAXIS 2023; 18(6): 40-41
InFo NEUROLOGIE & PSYCHIATRIE 2023; 21(4): 34–35