Iron deficiency is common in heart failure patients. In addition to a reduction in exercise capacity and quality of life, an increased risk of mortality is associated with it – regardless of whether anemia is present. The ESC guidelines therefore recommend routine collection of iron status. In a deficiency state, intravenous supplementation with ferric carboxymaltose has been shown to be more effective than oral iron.
80% of patients with heart failure are over 65 years of age and it is the second most common cardiovascular reason for consultation in a physician’s office after hypertension [1]. Iron deficiency is a comorbidity recognized by the European Society of Cardiology (ESC) in chronic heart failure, affecting 37-61% of patients [2]. More specifically, a Swiss study showed a 54.7% prevalence of iron deficiency in heart failure patients [11]. In a cohort study, iron deficiency has been shown to be an independent predictor of mortality in heart failure patients [3]. Improving the timely diagnosis and treatment of comorbid iron deficiency in heart failure is of major importance. One of the reasons that iron deficiency in cardiac inefficiency often goes undetected is that many of the symptoms overlap (decreased physical performance, fatigue/fatigue, restless sleep, cognitive difficulties). Another reason is that it is sometimes mistakenly assumed that testing for anemia is sufficient or that cut-off values are set too low when interpreting ferritin levels.
Iron status screening is essential – be sure to use adequate standard values!
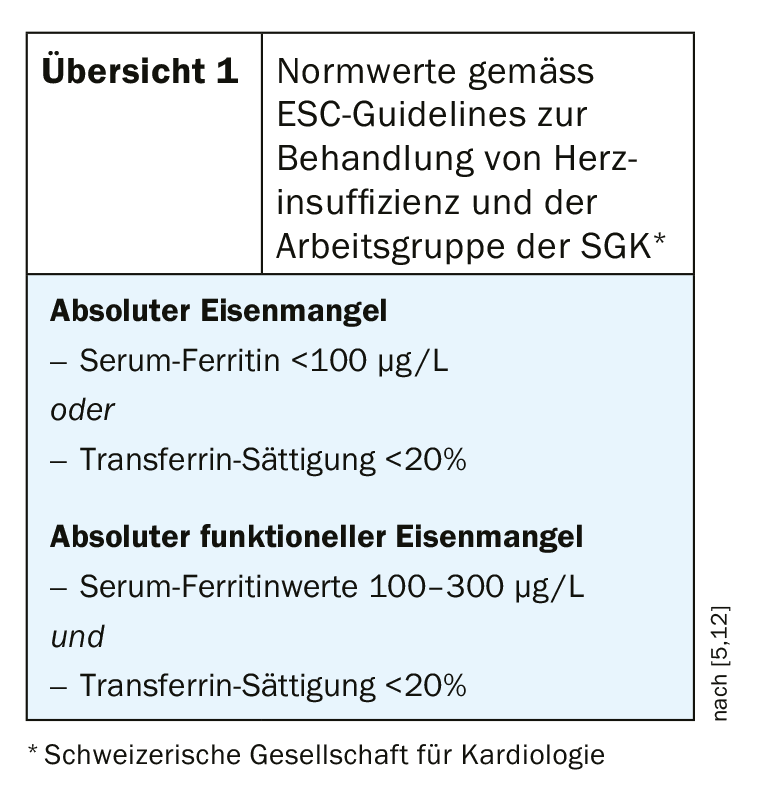
A clinically relevant iron deficiency is characterized by insufficient iron availability to meet the body’s needs, which, according to current knowledge, is possible whether or not anemia is present [4]. Even if hemoglobin (Hb) levels are within the normal range, iron deficiency may be present, which can lead to worsening heart failure and quality of life [2]. The ESC 2016 guidelines for the diagnosis and management of acute and chronic heart failure recommend iron status screening in all newly diagnosed patients with chronic heart failure [2,5]. It is also very important to use the appropriate norm values when interpreting the results (overview 1). The cut-off for heart failure patients is higher than that for other patients. According to ESC guidelines for the treatment of heart failure, the following standard values are authoritative for the diagnosis of iron deficiency: serum ferritin <100 μg/L or transferrin saturation <20%. If serum ferritin levels are in the range of 100-300 μg/L, a transferrin saturation test is required; if values <20% are obtained, functional iron deficiency is present.
Intravenous iron supplementation is more effective than oral supplementation
The ESC guidelines explicitly state to consider intravenous iron therapy with ferric carboxymaltose for the treatment of iron deficiency [2]. This is especially true for symptomatic patients with chronic systolic HFrEF or heart failure with LVEF <45% and iron deficiency [5]. In the placebo-controlled IRONOUT trial, oral iron supplementation (150 mg 2×/d) showed no effect on exercise capacity in heart failure patients (NYHA II-IV, LVEF ≤40%) after 16 weeks [6]. Moreover, oral iron did not result in an increase in ferritin levels and transferrin saturation compared with placebo. A major reason for this is that oral iron is poorly absorbed in heart failure patients. Therefore, the authors do not recommend that these patients first undergo prolonged oral substitution. However, observations show that this is contrary to current clinical practice, and most heart failure patients are treated with oral iron first, according to the professional information.
Ferric carboxymaltose: rapidly and persistently effective therapeutic option
The beneficial effects of intravenous iron supplementation have been empirically demonstrated. In the CONFIRM-HF, FAIR-HF, and EFFECT-HF randomized trials, intravenous supplementation with ferric carboxymaltose in patients with chronic heart failure and reduced left ventricular ejection fraction (HFrEF) resulted in improved symptoms, exercise capacity, and quality of life, as well as a reduction in hospitalization rates [7]. The administration of 1-2 intravenous iron infusions with ferric carboxymaltose (Ferinject®) [8,10] is completed in practice within approximately 45 minutes and the positive effects are quickly noticeable for the patient. With oral iron supplementation, the duration of treatment is usually 6-12 months and often treatment goals are missed. Iron takes over several important functions in the organism, so that a deficiency condition over a longer period of time becomes significant. Iron deficiency is associated with reduced transport and utilization of oxygen in the muscles. However, the function of iron in the heart goes beyond oxygen transport. Iron also plays an important role in ATP production in mitochondria as fuel for muscle contraction [8]. Cardiomyocytes contain many times more mitochondria than other cells. Iron is required by mitochondria for the stimulation of enzymatic reactions and for energy production by cardiomyocytes [7].
Implications for cardiomyocyte contractility.
Iron deficiency has been scientifically shown to impair cardiomyocyte contractility, and an in vitro has shown that this effect is reversible with timely treatment [9]. This was investigated using human embryonic cardiomyocytes (stem cells) deprived of iron by experimental intervention. Using the Seahorse Mito stress test, mitochondrial cellular respiration was measured and contractility was quantified by applying video analysis. The video sequences are downloadable under the QR codes in the box [9].

In addition, spectrophotometric enzyme assays were performed. Analysis of the data revealed that iron deficiency reduced cellular ATP levels by 74% (p<0.0001) and contractility decreased by 43% (p<0.05). Figure 1 shows cardiomyocyte contractility in iron deficiency and after corrected iron deficiency [9]. From this it can be seen that in induced iron deficiency a marked reduction in contractility of embroynal cardiomyocytes is measurable, but this is reversible [9]. It can be deduced that treatment of iron deficiency by intravenous supplementation is also relevant for cardiomyocyte contractility and this is an important implication of appropriate therapeutic measures, especially in heart failure patients.
|
Video sequences: contractility of myocytes |
Take-Home Messages
- Iron deficiency is a common comorbidity in chronic heart failure. Approximately 37-61% of patients are affected [2].
- The European Society of Cardiology (ESC) guidelines for the management of heart failure and the Swiss Society of Cardiology (SGK) working group on heart failure recommend screening for iron status in all newly diagnosed heart failure patients [5,12].
- Iron deficiency may also be present independently of anemia, which is why ferritin levels and transferrin saturation should definitely be measured separately [2]. When interpreting the results, the cut-off values for heart failure patients should be used, which are higher than for other patients [5].
- Timely iron supplementation can have a positive impact on heart failure and thus on patients’ quality of life. Intravenous iron replacement therapy has been shown to achieve better effects than oral replacement in heart failure patients [7].
Literature:
- Mohacsi P: Iron deficiency – an important comorbidity in heart failure. Prof. Paul Mohacsi, MD, FOMF Internal Medicine, 6/23/20.
- Lam CSP, et al. (on behalf of the IRON CORE Group): Iron deficiency in chronic heart failure: case-based practical guidance. ESC Heart Failure 2018; 5(5): 764-771.
- Klip IT, et al: Am Heart J 2013; 165(4): 575-582.
- Cappellini MD, et al: Am J Hematol 2017; 92: 1068-1078.
- Ponikowski P, et al: ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016; 37: 2129-2200.
- American College of Cardiology, www.acc.org/latest-in-cardiology/articles/2016/11/10/15/58/wed-1145amet-ironout-effect-aha-2016. Accessed January 22, 2017
- Gstrein C, Meyer MR, Pablo A: Swiss Med Wkly 2017; 147: w14453.
- Rosca MG, Hoppel CL: Mitochondria in heart failure. Cardiovasc Res 2010; 88(1): 40-50.
- Hoes MF, et al: European Journal of Heart Failure 2018; 20; 5: 910-919. https://onlinelibrary.wiley.com/doi/full/10.1002/ejhf.1154
- Specialist information Ferinject®, www.swissmedicinfo.ch
- Wienbergen H, et al: Usefulness of Iron Deficiency Correction in Management of Patients With Heart Failure [from the Registry Analysis of Iron Deficiency-Heart Failure (RAID-HF) Registry. Am J Cardiol 2016; 118: 1875-1880.
- SGK: Diagnosis and management of chronic heart failure. New edition 2019 based on ESC Guidelines 2016, Working Group “Heart Failure” of the Swiss Society of Cardiology (SGK), www.heartfailure.ch
HAUSARZT PRAXIS 2020; 15(8): 18-20