Mantle cell lymphoma (MZL) is an aggressive non-Hodgkin’s lymphoma that accounts for approximately 3-6% of all NHL cases. It has a poor prognosis, especially after failure of first-line therapy. Lenalidomide was approved as a new option in the refractory or relapsed setting in November 2014. How does it compare to the best alternative therapy? The corresponding study results were presented at the ASH Congress in San Francisco. There are also unanswered questions in initial treatment, which is why chemotherapy-free approaches are currently being tested.
Currently, the initial treatment of mantle cell lymphoma (MZL) is not standardized. Previous studies in this setting have focused primarily on chemotherapy, which is generally not curative. New therapeutic options with lower toxicity are in demand. A phase II trial presented at the ASH Congress therefore tested lenalidomide combined with rituximab as a potential first-line option. This biologic combination – but also lenalidomide alone – has been shown to be effective in relapsed cases of MZL (28% [1] alone and 57% [2] combined overall response rate). In Switzerland, the active ingredient has therefore been approved in the second-line setting since November 2014.
It has now been tested whether a chemotherapy-free approach is also possible and useful in the first-line setting. The multicenter phase II trial of 38 untreated MZL patients had overall response as the primary endpoint and progression-free and overall survival as secondary endpoints. The median age of participants was 65 and they were predominantly male. The disease was either stage three or stage four in all, and bone marrow involvement was found in 89%. 37% had an elevated LDH level. The Mantle Cell Lymphoma International Prognostic Index (MIPI) risk score was evenly distributed with 34% of patients at low risk, 34% at intermediate risk, and 32% at high risk. The index of the proliferation marker Ki67, which also has high prognostic relevance, was below 30% in the majority. Overall, the authors judged the study population to be typical in terms of both demographics and disease characteristics.
Induction: In an induction phase, lenalidomide was administered at a dose of 20 mg/d (first three weeks of a monthly cycle) for twelve cycles. Escalation to 25 mg was possible with tolerance. Rituximab was administered at the standard dose (375 mg/m2): weekly in the first cycle of four weeks and then once every second cycle for a total of nine doses.
Maintenance: In the maintenance phase until progression, the dose of lenalidomide was reduced to 15 mg starting at cycle 13. Rituximab continued to be administered once every other cycle.
What came out?
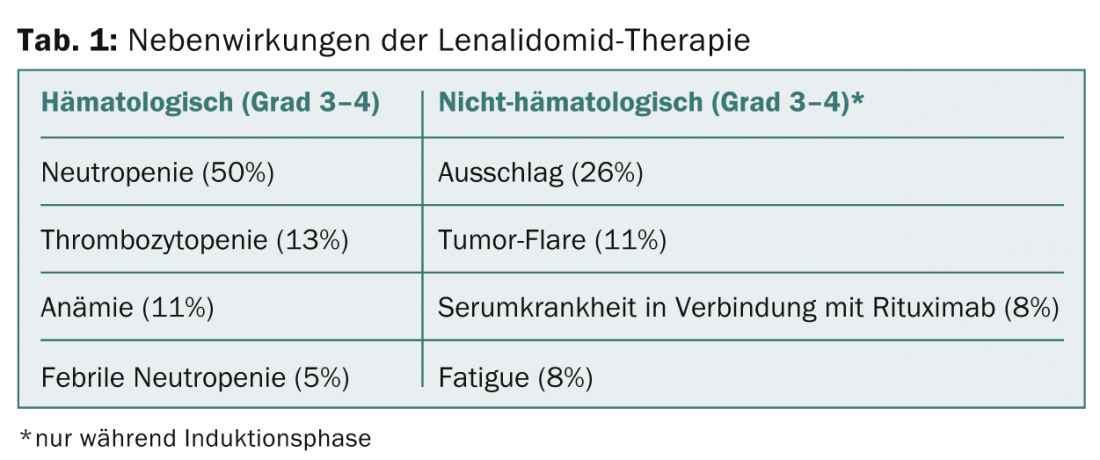
After a median follow-up of 26 months, the overall response rate of all evaluable patients (n=36) was a high 88.9%. Completely responded 58.3%, partially responded 30.6%. Progression-free survival at 24 months was 84.6% (95%CI 66.6-93.4%), and overall survival was 92.4% (95%CI 72.3-98.1%). In general, treatment was well tolerated and side effects were within expectations (Table 1). Grade 1-2 infections occurred during both treatment phases and included upper respiratory tract infections (40%), urinary tract infections (19%), and sinusitis (11%). Pneumonias of grade 3 or higher were found in 8%. Secondary tumors also occurred in 8%.
The authors conclude that the combination of lenalidomide and rituximab is active and safe, and thus may be considered as initial and maintenance therapy for MZL in the future. The non-cytotoxic approach achieves high response rates over a significant period of time as initial therapy. Quality of life remained stable or even increased during treatment. Further first-line research with both drugs – whether alone or in combination with other new agents – is therefore warranted.
MCL-002 study: lenalidomide in the second-line setting.
To date, while lenalidomide has shown activity in the refractory/relapsed setting in single-arm phase II trials (e.g., MCL-001 [1]) – leading to Swiss approval in November 2014 – it had not yet been proven how the drug compares to other treatment options.
MCL-002 is the first randomized-controlled phase II trial to compare lenalidomide dosed at 25 mg/d (first three weeks of a 28-day cycle until progression or intolerability) to the best treatment alternative. This could be single-agent therapy with cytarabine, gemcitabine, fludarabine for ≤6 cycles or rituximab or chlorambucil until progression. Crossover to lenalidomide was possible after disease progression (eventually 46% switching occurred).
The 254 patients were a median of 68.5 years old, mainly men, and had received two prior therapies. 91% were stage III/IV at diagnosis, 34% had high-risk MIPI, 43% had high tumor burden, and 20% had bulky disease. Overall, the lenalidomide group had a worse prognosis. Median progression-free survival (primary endpoint) was 8.7 months in the lenalidomide arm and 5.2 in the other arm. This corresponds to a significant risk reduction of 39% (HR 0.61, 95%CI 0.44-0.84, p=0.004). Also, the overall response rate, one of the secondary endpoints, and the complete response were significantly lower, with 40 vs. 11% and 5 vs. 0% significantly increased. Median overall survival was 27.8 months (lenalidomide) vs. 21.2 months (best alternative), missing significance (p=0.52). Overall, efficacy data were consistent across subgroups.
Side effect profile
The adverse event profile was as expected, with no new safety signals: third- or fourth-degree neutropenia (lenalidomide 44 vs. best alternative 34%, with no increase in infection rate), thrombocytopenia (18 vs. 28%), leukopenia (8 vs. 11%), anemia (8 vs. 7%), and febrile neutropenia (6 vs. 2%) were most common. Tumor flares (10%) were found only in the lenalidomide group. Secondary tumors were identified in 4 vs. 5%.
The authors conclude that the improvements with lenalidomide in the second-line setting are clinically meaningful, which is all the more surprising given that patients in this arm had a worse prognosis at baseline. The risk-benefit profile can be considered favorable. Also of note, median response duration has been remarkably consistent across multiple studies in the MZL setting to date (here it was 16.1 months).
Source: 56th ASH Annual Meeting, December 6-9, 2014, San Francisco.
Literature:
- Goy A, et al: Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol 2013 Oct 10; 31(29): 3688-3695.
- Wang M, et al: Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol 2012 Jul; 13(7): 716-723.
InFo ONCOLOGY & HEMATOLOGY 2015; 3(2): 22-23.