While the majority of studies on cardiac adaptation to sport have examined male athletes, there is growing evidence that female participation in competitive sports is greatly increasing. Therefore, it is important to recognize and understand gender differences in cardiac adaptation to ensure accurate medical care.
(red) The “athlete’s heart” is considered a physiological adaptation of the heart to regular exercise and is characterized by specific morphological and functional changes in the cardiovascular system. These adaptations affect both the left and right ventricles and in some cases can mimic pathological conditions such as cardiomyopathy. In recent years, non-invasive imaging techniques have contributed significantly to improving the assessment of cardiac adaptations in athletes. These advances help to better distinguish between physiologic adaptation and pathologic changes that could increase the risk of sudden cardiac death.
The “athlete’s heart”: gender-specific differences
Prolonged physical exertion, as occurs in competitive sport, leads to increased stress on the heart, which is referred to as the “athlete’s heart”. This adaptation manifests itself differently in male and female athletes. In men, concentric hypertrophy of the left ventricle (LV), characterized by a thickening of the LV walls, is more common. Studies have shown that about 15% of male endurance athletes develop concentric hypertrophy, while this is the case in only 4% of women. In contrast, female athletes are more prone to eccentric hypertrophy, which is characterized by an enlargement of the heart chambers.
Interestingly, this difference cannot be explained solely by the smaller body size and muscle mass of women. Even after adjusting for LV mass to body surface area (BSA), the differences between the sexes remain. Male athletes exhibit larger ventricular mass, while women show larger indexed ventricular dimensions. These observations suggest that hormonal and genetic factors play a crucial role in cardiac adaptation.
A key factor is the hormonal profile. From puberty onwards, men have significantly higher testosterone levels than women, and this difference persists throughout life. Testosterone and its metabolite dihydrotestosterone promote cardiomyocyte hypertrophy by increasing protein synthesis in cardiomyocytes. Antiandrogenic therapies have been shown to reduce pathological hypertrophy in the heart of animals. On the other hand, estrogen has a protective effect on the heart and inhibits the development of hypertrophy. In addition, estrogen plays a key role in oxidative energy production from fats, which leads to preferential fat burning in women during exercise, whereas men mainly oxidize carbohydrates. These differences may be related to the biological role of women as potential reproductive partners, which requires a more sparing use of carbohydrates.
Electrocardiographic differences in male and female athletes
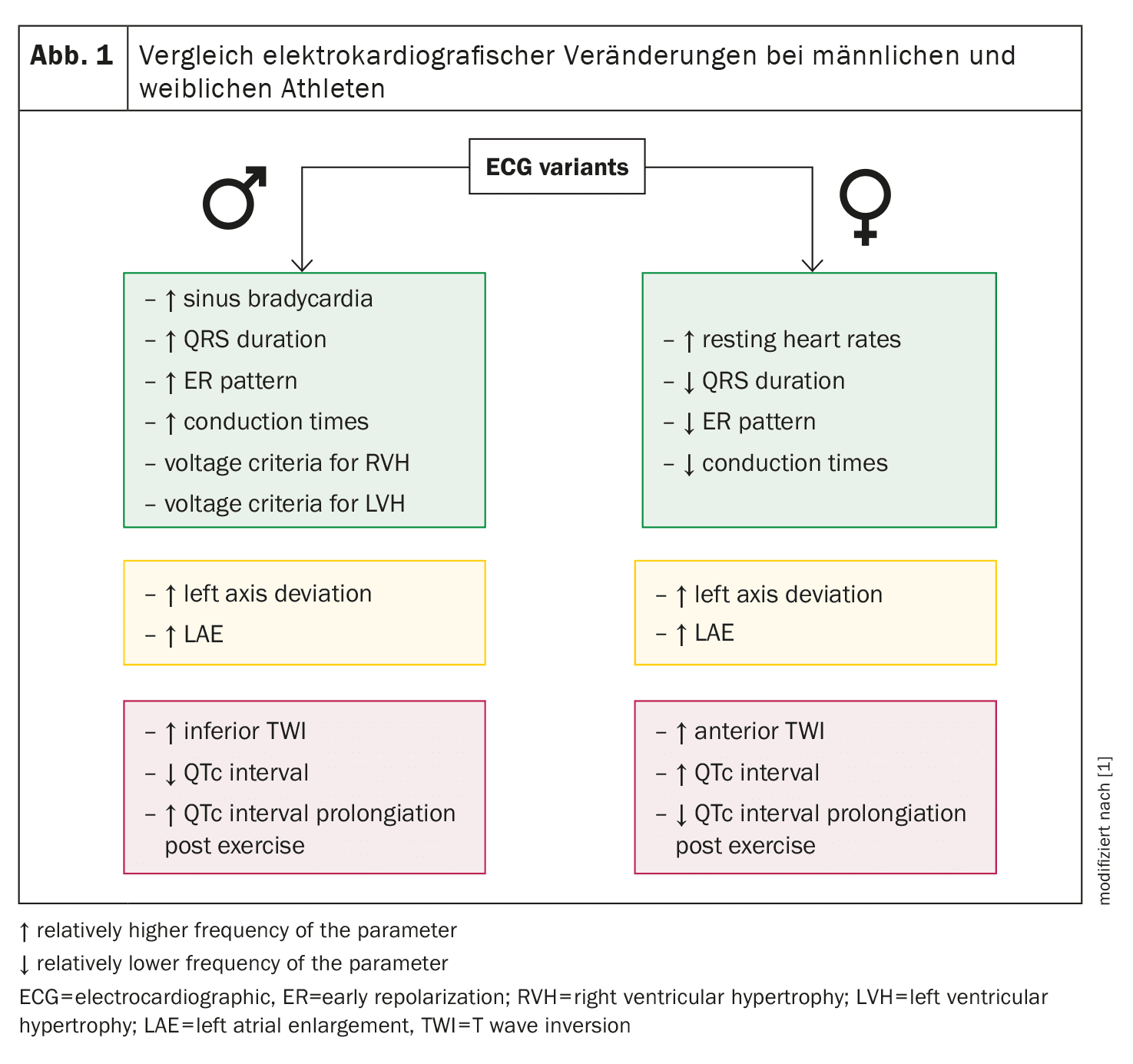
Electrocardiography (ECG) is an important non-invasive method for assessing cardiac adaptations in athletes. Over the years, specific criteria have been developed to differentiate between physiologic ECG changes caused by exercise and pathologic patterns that could increase the risk of sudden cardiac death. These criteria have been revised several times to improve the accuracy of the diagnosis and reduce the number of false positives.
Male athletes more frequently show ECG changes that indicate increased stress on the heart, such as sinus bradycardia, incomplete right bundle branch block (RBBB) and criteria for left ventricular hypertrophy (LVH). Female athletes, on the other hand, are less likely to exhibit these typical adaptations. An interesting observation is that women generally have a higher resting heart rate than men, which leads to a lower incidence of bradycardia. In addition, men show more frequent T-wave inversions in the ECG, especially in the inferior leads, which could indicate greater cardiac stress.
Another important difference concerns the QT interval. Women generally have longer QT intervals than men, which increases the risk of arrhythmic events. This prolongation of the QT interval in women is due to hormonal differences and may explain why women have a lower risk of sudden cardiac death compared to men. In fact, studies show that the incidence of sudden cardiac death is significantly higher in male athletes than in women. Nevertheless, QT time should be monitored in both sexes as part of regular cardiac examinations in athletes, as prolonged QT intervals are a significant risk factor for malignant arrhythmias and sudden cardiac death.
Echocardiographic adaptations and gender-specific differences
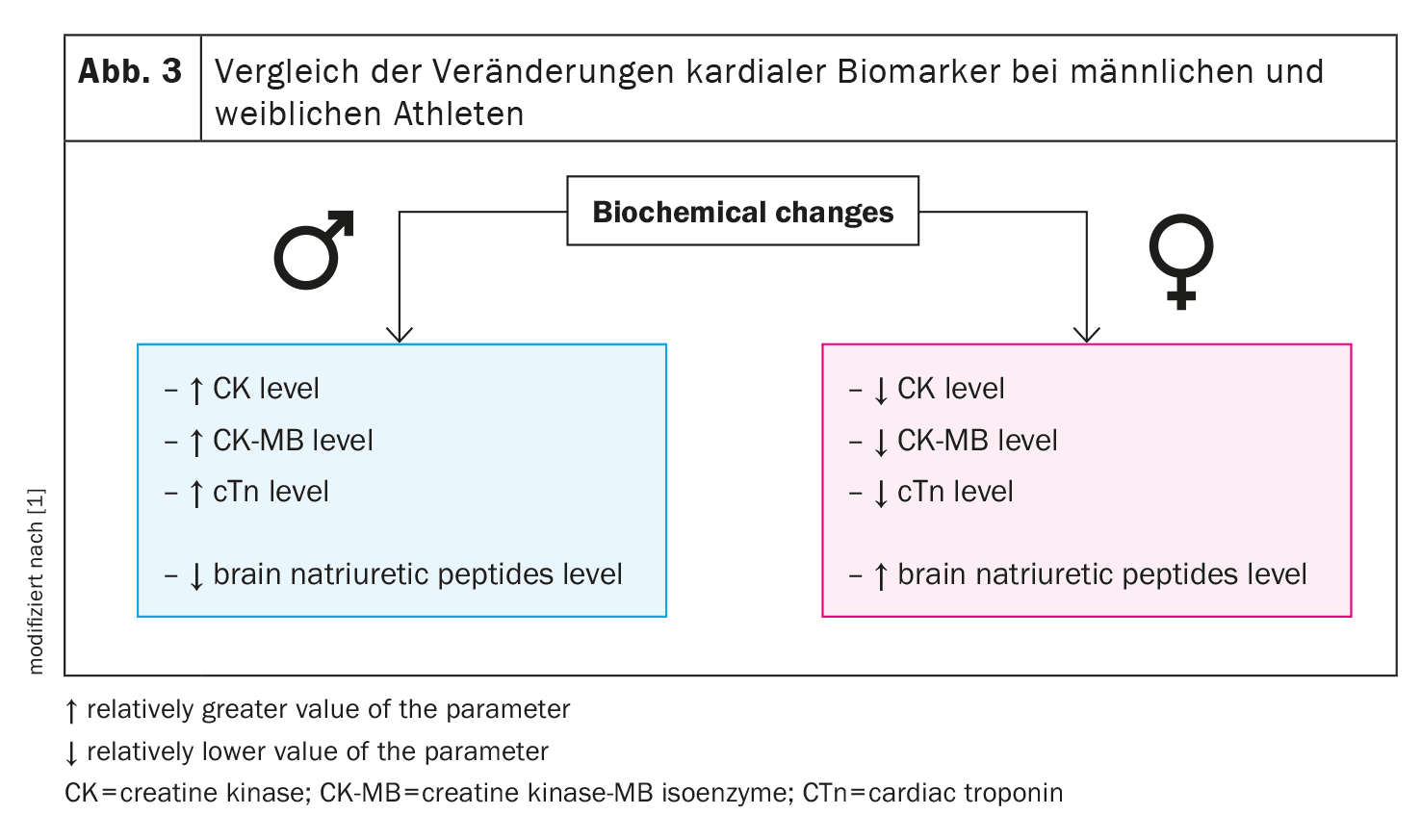
Echocardiography offers an extremely precise and non-invasive way of assessing cardiac structure and function in athletes. Long-term physical exertion generally leads to an enlargement of the heart chambers, especially the left ventricle. Men tend to have concentric hypertrophy, in which the wall thickness of the left ventricle increases, while women tend to have eccentric hypertrophy, in which the ventricular cavity enlarges.
Studies show that male athletes are more likely to develop concentric left ventricular hypertrophy, while the wall thickness of the right ventricle and left ventricle is greater in women despite lower body mass in relation to body surface area. This suggests that women may experience greater structural adaptation of the left ventricle, which may reflect the greater flexibility of the female heart to adapt to increased volume loading.
In addition to the morphological adaptation of the ventricles, echocardiographic examinations show differences in systolic and diastolic function. Men generally exhibit a greater strain on the heart muscles, which can lead to a greater decline in systolic function after intensive training sessions. This change is often due to a stronger activation of the sympathetic nervous system, which is more dominant in men, while women have a stronger parasympathetic regulation of the heart. This difference could explain why men more frequently experience a deterioration in systolic function after intensive endurance exercise.
In diastolic function, women show a higher E-wave velocity compared to men, which indicates a more efficient relaxation phase of the left ventricle. However, after intensive training sessions, such as a marathon, a decline in diastolic function is observed in both sexes, although this effect is more pronounced in men. Studies have shown that the loss of diastolic function after intense exercise tends to be lower in women due to higher oestrogen levels, suggesting a protective effect of the hormone.
Biochemical markers of cardiac adaptation in men and women
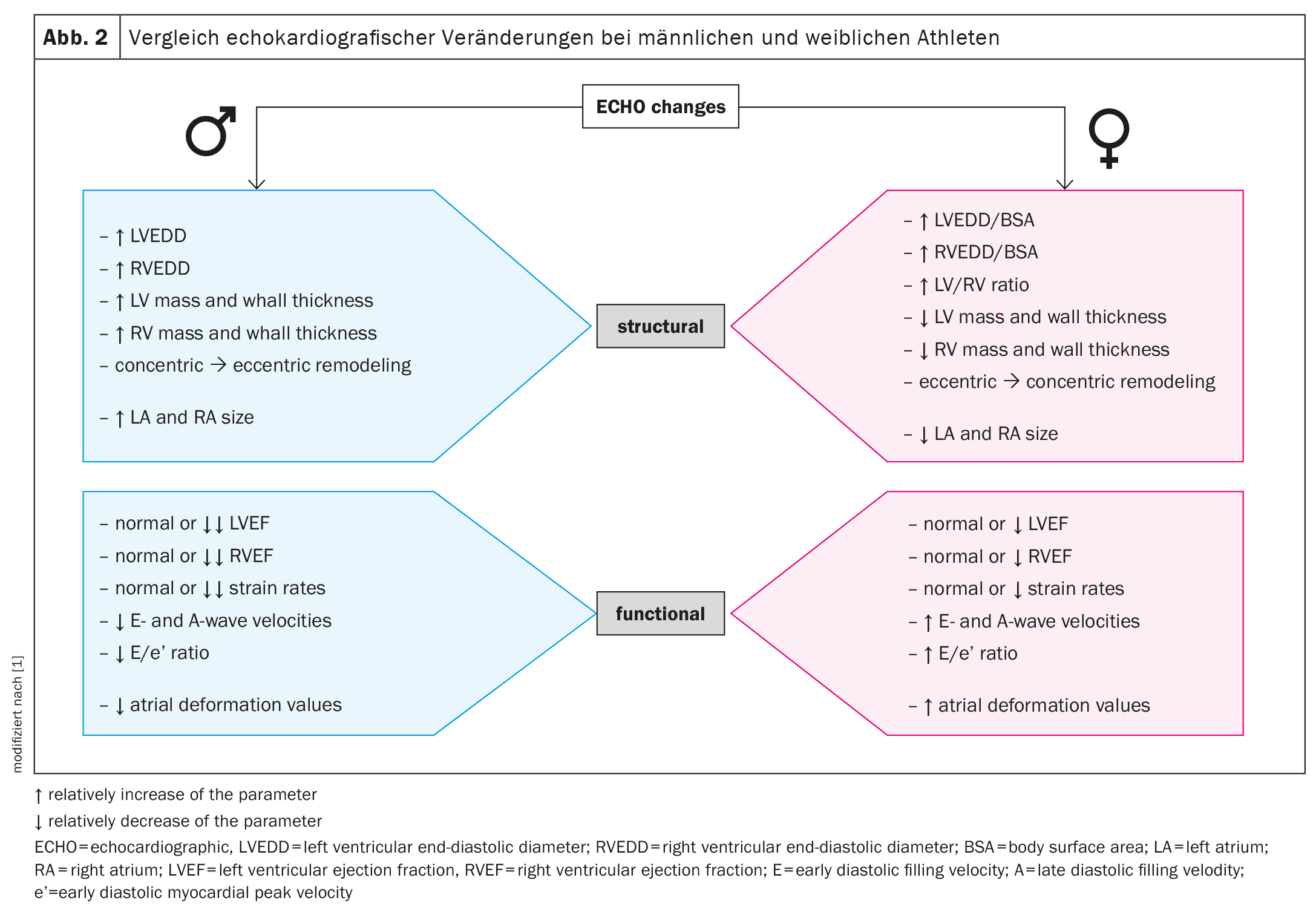
In addition to structural and functional adaptations, biochemical markers can also provide valuable information about cardiac adaptation to physical stress. Intense physical activity leads to a transient increase in biomarkers such as cardiac troponin (cTn) and blood natriuretic peptide (BNP), both of which indicate myocardial overload. These biomarkers are often used as indicators of cardiac damage, but can also increase as a normal response to intense exercise.
Male athletes generally show higher resting cTn values compared to women. This could indicate the larger heart mass of men and the associated greater strain on the heart. After intensive training sessions, such as a marathon, men show a significantly higher increase in cTn values than women. This difference can be explained by hormonal differences and the associated metabolic and cardiac adaptations.
BNP and NT-proBNP, which serve as markers for wall tension and volume overload of the heart, increase in both male and female athletes after intense physical exercise. Interestingly, the increase in BNP levels is more pronounced in women, which may be due to the higher estrogen levels in women. Estrogen stimulates the synthesis of natriuretic peptides, which could explain a stronger hormonal response to cardiac stress in women.
In addition to the known biomarkers, new markers such as growth and differentiation factor 15 (GDF-15) and heart-type fatty acid-binding protein (H-FABP) are also being investigated as potential indicators of transient cardiac damage after intense exercise. These markers increase transiently after intense endurance exercise and usually normalize within 72 hours. However, the gender-specific difference in the response of these biomarkers has not yet been fully explored and requires further study.
Clinical relevance and future prospects
Understanding the gender-specific differences in cardiac adaptation to physical stress is of great clinical importance. The diagnosis of the “athlete’s heart” and the differentiation between physiological and pathological changes are essential for the cardiological care of athletes. Male athletes in particular are at increased risk of developing cardiac diseases that can lead to sudden cardiac death. Therefore, regular cardiac examinations, including ECG, echocardiography and biochemical tests, should be performed to detect potential risks at an early stage.
Women generally show less cardiac adaptation to physical exertion, which makes them less susceptible to certain cardiac complications. Nevertheless, regular monitoring is also important in female athletes, as hormonal changes, especially after the menopause, can lead to a deterioration in cardiac function.
Overall, the current studies show that both male and female athletes have specific cardiac adaptations to physical stress. However, further research is needed to fully understand the underlying mechanisms of these gender differences and to develop personalized approaches to cardiac care for athletes.
Source:
- Lasocka-Koriat Z, Lewicka-Potocka Z, Kaleta-Duss A, et al: Differences in cardiac adaptation to exercise in male and female athletes assessed by noninvasive techniques: a state-of-the-art review. Am J Physiol Heart Circ Physiol. 2024 May 1; 326(5): H1065-H1079. doi: 10.1152/ajpheart.00756.2023. Epub 2024 Feb 23. PMID: 38391314; PMCID: PMC11380999.
CARDIOVASC 2024; 23(3): 28-30