With state-of-the-art genetic testing, diagnoses can now be made that would otherwise not be possible. In addition, genetic clarification has significance for family counseling as well as for pharmacological treatment. But be careful: not all genetic tests are the same.
The steady progress in human genetics, comparable to the exponential development of computer technology, is leading to the identification of the cause of more and more genetic diseases. Thus, today a diagnosis can increasingly be made, confirmed or ruled out by means of genetic testing (genetic testing) of the genetic material (DNA). Such genetic tests for medical purposes (genetic diagnostics), on which this article focuses, are not to be confused with (lifestyle) genetic tests for non-medical purposes from the Internet or the pharmacy (Fig. 1).
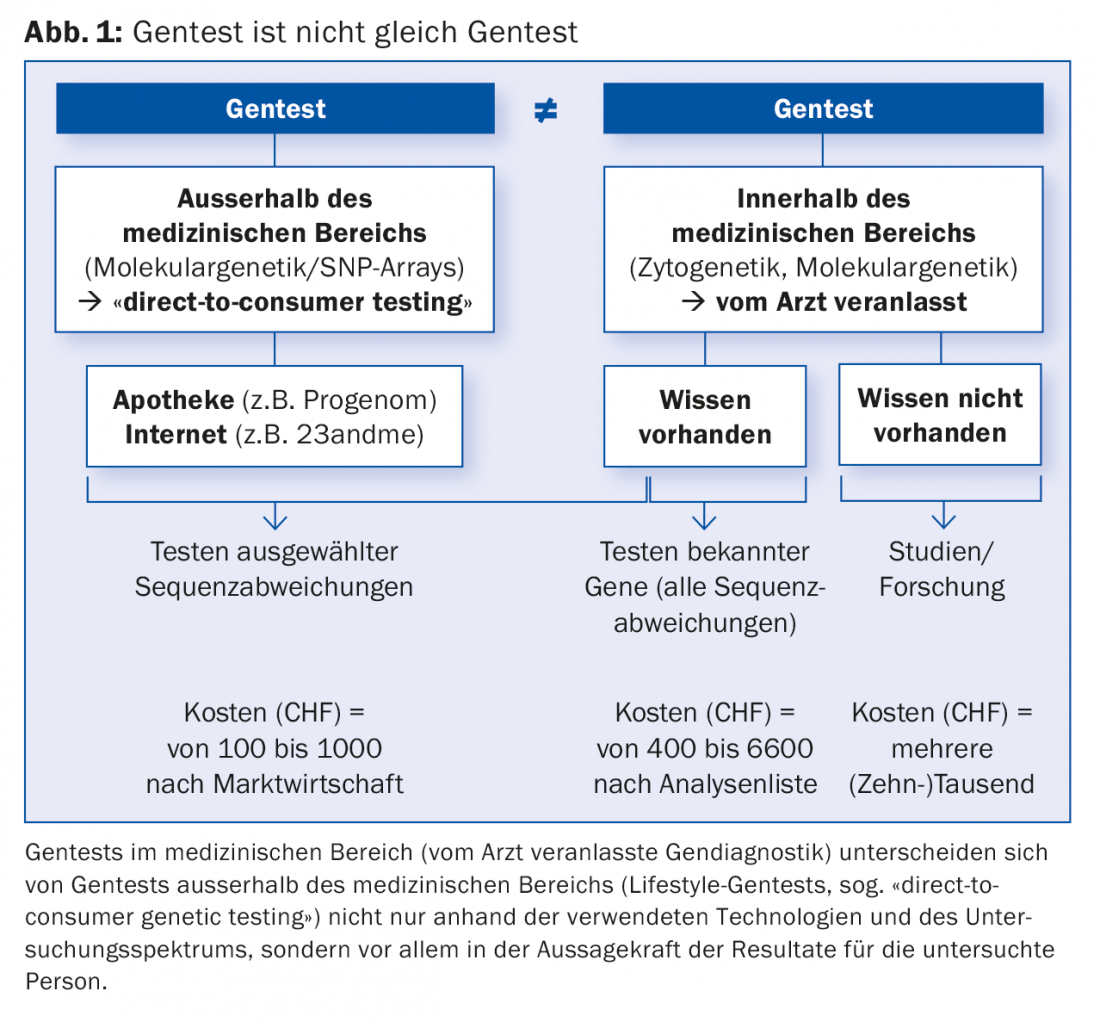
Increasing importance of genetic diagnostics
Monogenic diseases are caused by the mutation of a single gene, whereas in common multifactorial diseases the (genetic) influences are usually multiple and only strong in sum. Genetic diagnostics can be performed pre- or postnatally by examining chromosomes (cytogenetics) and/or genes (molecular genetics) and is mainly used in situations where clinical examinations do not allow a conclusive diagnosis. This is especially important in the early stages of a disease and in children and adolescents. If the disease-causing mutation is known, it can also be determined pre-symptomatically in blood relatives whether or not there is a genetic predisposition to the familial disease. It should be noted that genetic workup of a late manifesting disease and carrier status of a recessive disease is reserved for adults.
Diagnosis of the underlying genetic defect allows targeted disease management and in some cases therapeutic strategies can be tailored to the individual causative mutation. Research is constantly searching for new tailor-made medicines that take into account the individual genetic characteristics of patients (“personalized/precision medicine”). The importance of pharmacogenetics, which enables the selection and dosage of an appropriate drug, is also steadily increasing, as it can help to avoid side effects, unnecessary loss of time in drug adjustment, and related costs.
High-throughput genetic diagnostics
The most important method for the targeted investigation of genes is DNA sequencing, which can determine the sequence of nucleotide bases of the genetic material (A, T, G, C) and thus precisely detect gene mutations. Such genetic analyses are performed with unprecedented efficiency using high-throughput sequencing (“next generation sequencing”, NGS). NGS is more efficient than classical single-gene analysis using Sanger sequencing and is particularly successful in detecting the causes of disease as well as analyzing cell-free DNA circulating in the blood and single-cell examination of minute biospecimens.
In NGS, either a selected combination (so-called panel) of genes (“targeted sequencing”, TS), the whole genome (“whole genome sequencing”, WGS; ~3 billion nucleotide bases) or its coding region (“whole exome sequencing”, WES; ~20 000 genes) is examined. For this reason alone, not all NGS is the same (Fig. 2) . In addition, there is the difference in performance and quality between the NGS methods, which is relevant for genetic diagnostics.
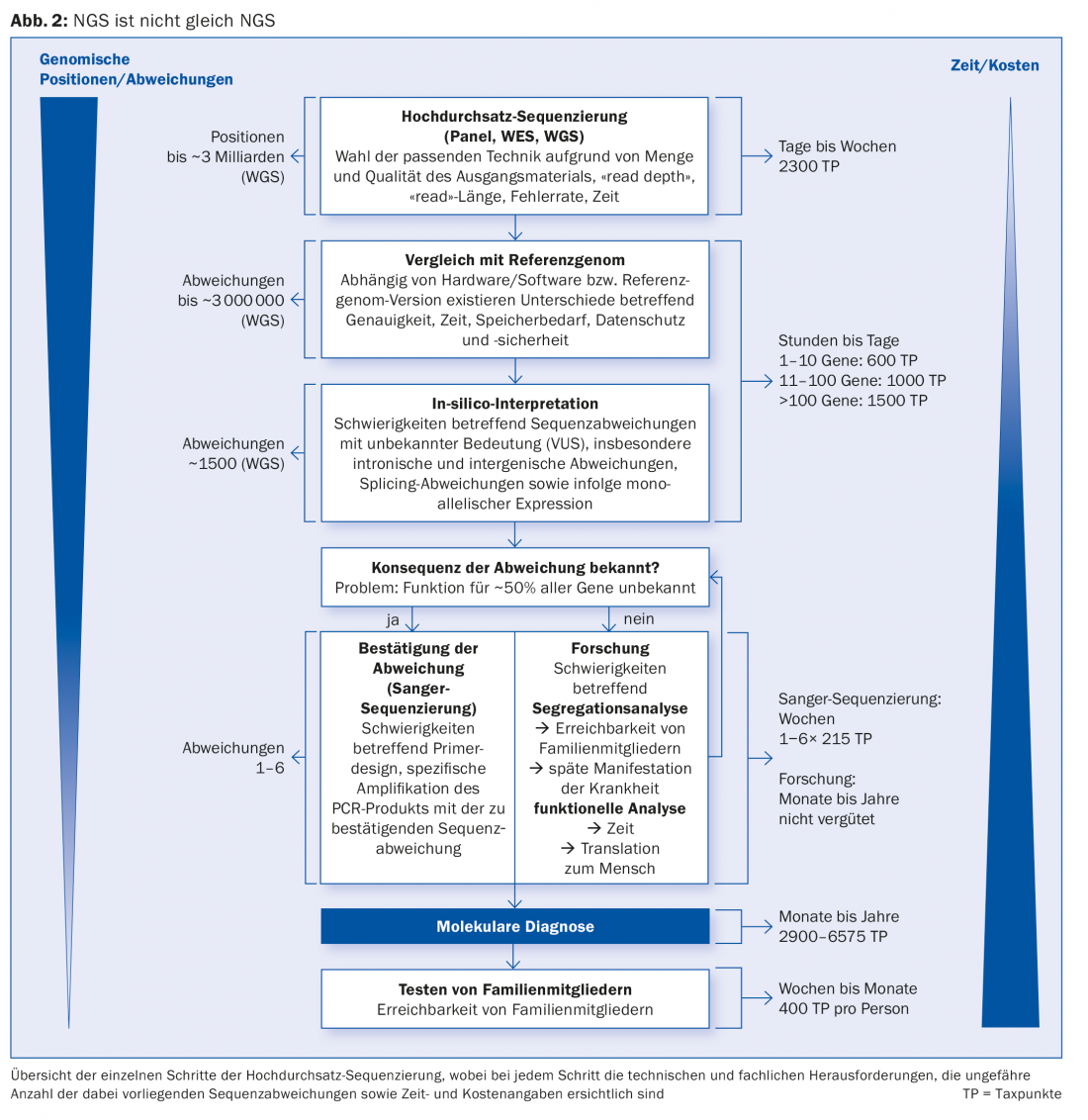
TS can analyze certain gene regions particularly intensively by capturing the sequence of more than 1000 DNA copies (“reads”) and thus small amounts (<1:100) of non-reference alleles, which are present as a so-called mosaic, can be detected. This is particularly necessary for genetic testing of somatic cancers. TS is also considered cost-effective and is therefore often used for the first step in mutation screening. However, if the disease-causing mutation is not found with TS, the disease remains undiagnosed and testing must be repeated with WES or, better yet, WGS. Caveat: A negative finding in genes associated with the disease under investigation at the time of workup does not mean that the disease cannot be genetic.
Challenges of high-throughput sequencing
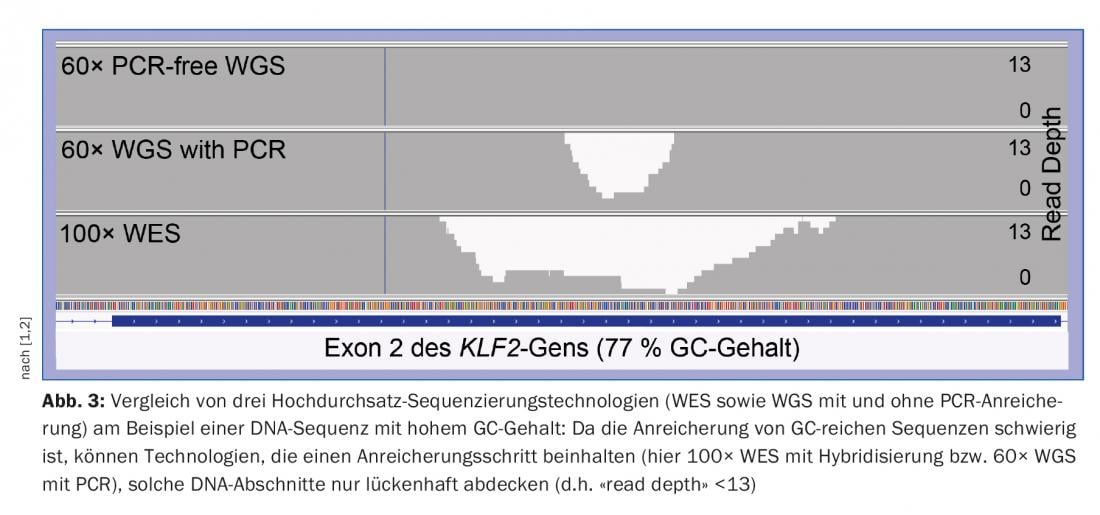
There are important limitations with NGS from a genetic diagnostic perspective. First, the sequence read length of Illumina’s market-leading NGS technology is too short (~150 nucleotide bases) to assign longer repetitive/homologous gene regions to the reference genome with unique location. The latest sequencing technologies (“third generation sequencing”), e.g. from Pacific Biosciences or Oxford Nanopore Technologies, which can read significantly longer DNA fragments of several thousand nucleotide bases, promise a remedy. Second, NGS of GC-rich DNA regions is more difficult because the nucleotide base pair G and C has stronger binding than the pair A and T. Especially in TS and WES, GC-rich gene regions, as in many cases at the beginning of a gene, are not sufficiently covered (i.e., not sufficiently covered with “sequencing reads”), which is why the quality requirements of genetic diagnostics are often not sufficiently met [1]. With WGS, this problem occurs much less, so that WGS not only has the advantage of covering the non-coding region of the genome, but also covers the clinically particularly important coding regions (exons) better than WES, especially for GC-rich regions (Fig.3). WGS is thus superior to WES and currently allows the best possible genetic diagnosis of congenital diseases whose causes are unknown or which are based on mutations in large and complex genes [2].
The large amount of data (Big Data) generated in the context of a WGS can be reduced to the level of TS and WES with virtual (in silico) gene panels and focused on the clinical question. In addition, quality control and interpretation of the amount of data generated by NGS is an elaborate challenge, both costly and intellectual, which is not yet adequately reflected by the analysis list (AL) with the new position for NGS in place since 2015. Especially the acquisition and interpretation of those NGS data that are important for diagnostics require a lot of human genetic expertise and can be particularly time-consuming. This is the case, for example, when potentially pathogenic sequence deviations are found in genes whose function is not yet (fully) known – which is still the case for about half (~10 000) of all human genes today.
Although today’s interpretation software takes into account many different parameters and provides important bases for assessment, the interpretation of such sequence deviations (so-called “variants with unknown significance”, VUS) with diagnostic certainty requires at least an elaborate segregation analysis in the family (if possible) and/or functional assays/studies (e.g. using an appropriate animal model).
Clinically relevant incidental findings
Before a genetic examination, it must also be discussed with the person to be examined whether any incidental findings with clinical significance but unrelated to the research question (so-called “incidental findings”) should only be reported if preventive or treatment measures are known [3]. This alone shows that the results of a genetic examination are not comparable with other medical laboratory tests and that their evaluation belongs in the expert hands of specialists in medical genetics FAMH/FMH. The current analysis list therefore stipulates that NGS of more than ten genes may only be prescribed by physicians with a federal postgraduate qualification in “Medical Genetics”. Also, the genetic counseling required by law before and after genetic testing should be provided by medical geneticists (GUMG Art. 14). Counseling may only take into account the individual and family situation of the person concerned and not general social interests. It must take into account the possible psychological and social impact of the examination result on the person concerned and his or her family.
Reimbursement by basic insurance
Genetic diagnostics is a particularly responsible activity. Genetic findings usually affect not only the person under investigation throughout his or her life (“lifetime value”), but can also have an impact on the entire family over generations. Incorrect or missing diagnoses can lead to mistreatment with serious consequences, to psychological stress and unnecessary limitations, or to the birth of more severely affected children. Beyond the burden or illness of individual patients and families, this can also result in significant economic costs. In contrast, the combined share of all genetic and laboratory diagnostic analyses accounts for just about 3% of the total annual cost of our health care system.
The analysis list (www.bag.admin.ch) regulates the payment for NGS, which may only be charged if the cost of classical single gene analysis using Sanger sequencing would be higher than 2795 tax points (TP), which applies to genes with more than 13 target sequences (exons). The tariff for NGS is composed of the actual high-throughput sequencing (2300 TP) and the bioinformatic analysis for 1-10 genes (600 TP), for 11-100 genes (1000 TP) or for more than 100 genes (1500 TP). Together with the confirmatory investigation of positive NGS results, the analysis list provides for a remuneration of 2900 TP to 6575 TP, which, however, is not cost-covering for the genetic clarification of clinically complex (rare) cases up to the diagnostic endpoint. It is hard to believe that this gap in the Swiss health care system has to be closed by the commitment of the population and private organizations (cf. stiftung-seltene-krankheiten.ch). In addition, the genetic tests explicitly listed in the analysis list are arbitrarily rejected by several health insurance companies, so that there is also an urgent need for action here. The reimbursement of genetic testing for medical purposes must be improved so that the clinically highly relevant developments in the field of human genetics can be translated into diagnostics.
Outlook
In the future, genetic diagnostics will be crucial – not only for the detection of disease-causing genetic defects, but also for the pharmacogenetic analysis of genes that help determine the pharmacokinetics and dynamics of the drugs used and thus their selection and dosage. Furthermore, the medical importance of detecting somatic gene mutations will increase. Increasingly, (epi)genetic changes that do not directly affect the DNA sequence of genes, but rather their regulation, are also being studied and understood. Strong genetic effects can also be seen in common multifactorial diseases.
New methods such as CRISPR/Cas open up possibilities, but also dangers, in the treatment of genetic diseases. Despite promising results of in vivo experiments so far, this technology is still far from finding its way into everyday medical practice. On the one hand, because the method is not yet fully developed, and on the other hand, because ethical and legal issues need to be discussed in order to limit any potential for abuse.
Nevertheless, it is obvious that genetics will determine the future of medicine and that medicine will be guided by genetics in prevention, diagnosis and therapy.
Take-Home Messages
- Not all genetic tests are the same: The methods used determine the significance of each test.
- With appropriate state-of-the-art genetic tests, diagnoses can now be made that would otherwise not be possible.
- Familial and/or early or syndromal occurrence of a disease may indicate a genetic cause.
- Genetic workup has importance not only for diagnosis, prognosis, prevention and family counseling, but also for causal or pharmacological treatment.
Literature:
- Meienberg J, et al: New insights into the performance of human whole-exome capture platforms. Nucleic Acids Res 2015; 43: e76.
- Meienberg J, et al: Clinical Sequencing: Is WGS the better WES? Hum Genet 2016; 135: 359-362.
- Kalia SS, et al: Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med 2017; 19: 249-255.
Further reading:
- Henggeler C, Matyas G: Ways of diagnostics – genetic testing for medical purposes. HAUSARZT PRAXIS 2015; 10(3): 4.
- Matyas G, Henggeler C, Oexle K: Cardiovascular genetics and genetic diagnostics. Medinfo 2015; 2: 16-24.
- Matyas G, Spiegel R: Genetic clarification for medical purposes. Medinfo 2012; 2: 50-58.
- Oexle K, Henggeler C, Matyas G: The increasing importance of genetics in medicine. HAUSARZT PRAXIS 2015; 10(9): 9.
HAUSARZT PRAXIS 2017; 12(9): 14-18











