Not only because of its high mortality, infective endocarditis requires comprehensive treatment. Close interdisciplinary cooperation is necessary to achieve the best possible results.
Infective endocarditis is a complex disease associated with high mortality. Close interdisciplinary cooperation, especially between cardiologists, infectiologists, radiologists, and cardiac surgeons, is necessary to ensure the best possible treatment. Publications of new articles, improvements in imaging techniques, and discrepancies between previous European and U.S. guidelines led to an update of the European guidelines in 2015 [1]. This article summarizes these guidelines.
Prevention
The indications for performing endocarditis prophylaxis were already restricted several years ago. Everyday activities, such as tooth brushing, cause repetitive, low-level bacteremia, which probably poses a higher risk for the development of endocarditis than the infrequent bacteremia associated with (dental) surgery [2]. Therefore, antibiotic endocarditis prophylaxis is thought to prevent only a small number of endocarditis cases, if any. Endocarditis prophylaxis is therefore now recommended only for patients at highest risk for acquiring and for an unfavorable course of endocarditis. According to the European guidelines, these are patients with prosthetic valves or with reconstructed valves (if prosthetic material is used), patients after having undergone endocarditis, or patients with cyanotic, palliative care, or congenital vitiation corrected by foreign material (for 6 months postintervention) [1]. The Swiss guidelines additionally recommend prophylaxis for patients with a ventricular septal defect, a persistent ductus botalli, or for patients with valvular vitiation in transplanted hearts. While the European guidelines only provide for prophylaxis before dental procedures, Switzerland also recommends prophylaxis before certain procedures on the gastrointestinal, urogenital and respiratory tracts [3].
Diagnosis
Clinic, microbiology (especially blood cultures) and echocardiography represent the cornerstones in the diagnosis of endocarditis. In addition, other imaging techniques can provide important information.
Clinic: The clinical picture of endocarditis is variable and depends on the a.o. depends on the pathogen and various patient characteristics. The most common symptom is fever, which occurs in up to 90% of cases. A heart murmur can be auscultated in up to 85% of cases. Embolic complications are found in approximately 25% of patients at diagnosis [1]. The classic peripheral stigmata of vascular (e.g., Janeway lesions, splinter hemorrhages) and immunologic (e.g., Osler nodules, Roth spots) etiology have become less common because they are v.a. are present in subacute cases and nowadays patients tend to present in the acute stage of endocarditis.
Microbiology: If endocarditis is suspected, at least three pairs of blood cultures should be obtained from peripheral veins at 30-minute intervals before starting antibiotic therapy. In nearly 1/3 of endocarditis cases, these blood cultures are negative. The most common reason for this is prior antibiotic therapy. In such situations, an antibiotic-free time window followed by repeat blood culture collection is indicated, if acceptable from a clinical standpoint. Other causes of negative initial blood cultures include fungi, and difficult-to-culture or intracellular bacteria (e.g., Coxiella burnetii, Bartonella spp., Brucella spp.). If initial blood cultures are negative and endocarditis is persistently suspected, cultures on special media, serologic testing, or “polymerase chain reaction” testing can help establish the diagnosis [4]. If surgery is performed on an infected valve, microbiological examination of the resected material is of diagnostic importance.
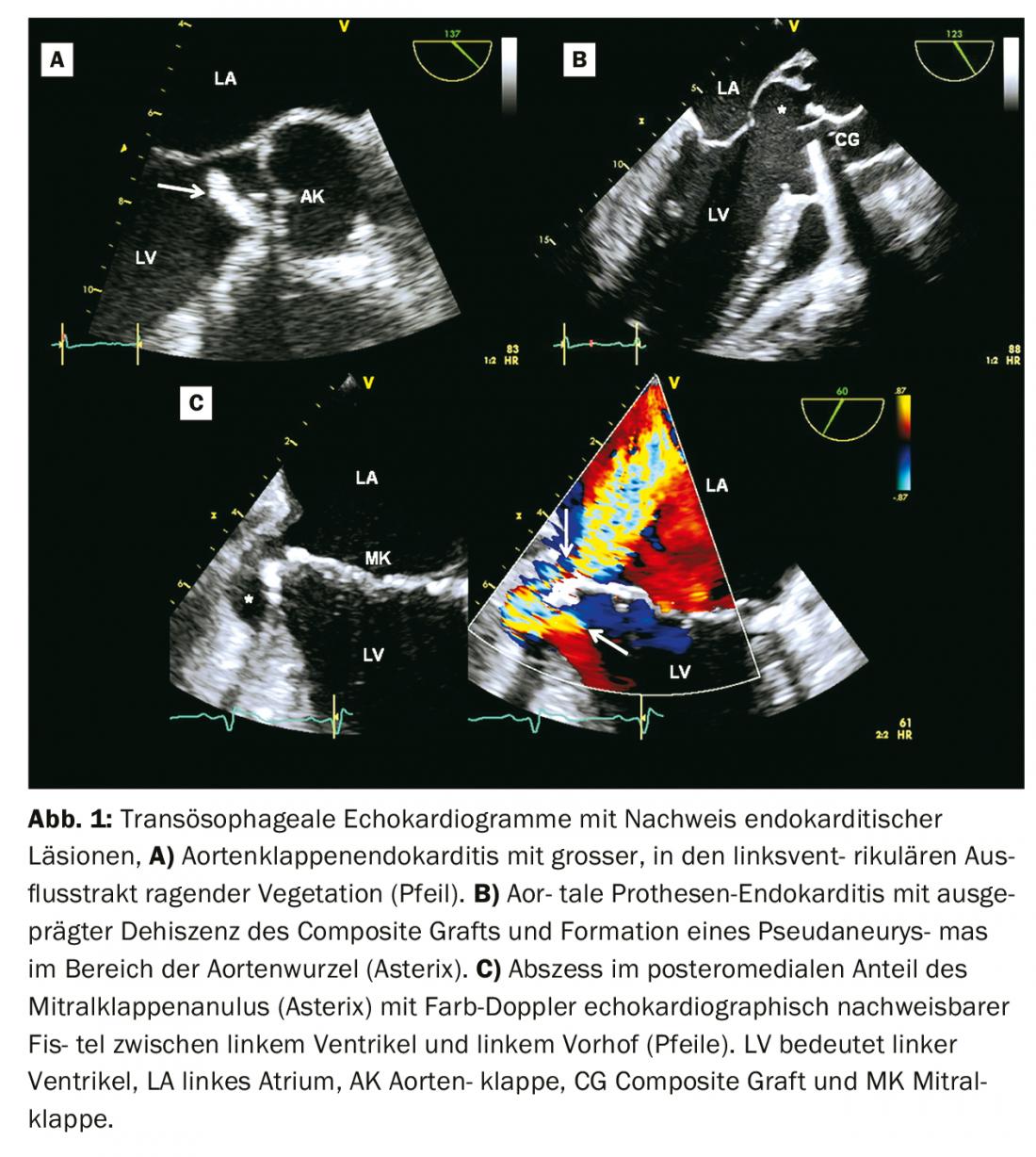
Echocardiography: echocardiography is the imaging of first choice in suspected endocarditis [5]. Usually, transthoracic echocardiography (TTE) is performed first. A transesophageal echocardiogram (TEE) is usually indicated as a follow-up examination, except in cases of negative TTE and clinically low suspicion of endocarditis. According to both Duke’s criteria and the modified diagnostic criteria of the European guidelines, vegetations, paravalvular complications (abscess, pseudaneurysms, fistulas), or new dehiscence of a prosthetic valve are considered echocardiographic major criteria for endocarditis (Fig. 1, Tab. 1) [1,6]. In the modified diagnostic scheme of the European guidelines, newly detected valvular insufficiencies are no longer considered a diagnostic sign, whereas valvular perforations and valvular aneurysms are considered major criteria (Table 1) [1].

The sensitivity for diagnosing vegetations by TTE is 70% in native valves and 50% in prosthetic valves. For the TEE, the corresponding values are 96 and 92%, respectively. Specificity is approximately 90% for both modalities [5]. The sensitivity for diagnosing abscesses in TTE is 50% and in TEE 90% [5].
If initial echocardiographic evaluation reveals no diagnostic findings and a high clinical suspicion of endocarditis persists, repeat TTE and TEE after 5-7 days is recommended [1]. In addition, the performance of complementary imaging techniques should be considered in these situations, especially in echocardiographically challenging cases such as patients with prosthetic valves or with intracardiac electronic devices.
Other imaging modalities: Cardiac computed tomography (CT) may be helpful in diagnosing paravalvular complications (Table 1) [1], especially in prosthetic endocarditis. CT is also useful for detecting extracardiac complications of endocarditis (e.g., ischemic lesions due to embolization of a vegetation, abscesses). Magnetic resonance imaging (MRI) is particularly useful for detecting cerebral lesions. Cerebral lesions are frequently found and are usually embolic-ischemic in nature. Even neurologically unremarkable patients show lesions on MRI in at least 50% of cases [7]. In the modified diagnostic scheme of the European guidelines, paravalvular lesions on cardiac CT are considered major criteria and vascular com- plications detected by imaging (CT or MRI) are considered minor criteria (Table 1) [1].
Nuclear imaging techniques, such as 18F-FDG PET/CT or leukocyte SPECT/CT, can also be helpful in diagnostically difficult cases. According to recent data, these studies may improve the diagnostic accuracy of endocarditis in prosthetic valves and intracardiac devices [8]. However, when evaluated by 18F-FDG PET/CT, valve surgery must have occurred at least 3 months ago, because nonspecific metabolic activity in the area of the prosthesis is possible shortly after surgery. SPECT/CT is more specific than 18F-FDG PET/CT in detecting endocarditis [9]; however, it is a complex, time-consuming imaging technology.
In the modified diagnostic scheme of the European guidelines, the detection of abnormal periprosthetic activity in nuclear imaging is considered a major criterion (Table 1) [1].
Therapy
Antibiotic therapy: Antibiotic therapy is administered intravenously. The duration depends on the pathogen and the complexity of the endocarditis. For native valves, initial empiric therapy with amoxicillin 6 × 2 g i.v., gentamycin 3 mg/kg i.v. and fluocloxacillin 6 × 2 g i.v. daily, in native valves and penicillin allergy with vancomycin 2 × 15 mg/kg i.v. and gentamicin 3 mg/kg i.v. daily and for prosthetic valves with vancomycin 2 × 15 mg/kg i.v., gentamicin 3 mg/kg i.v. and rifampicin 2 × 450 mg p.o. daily is recommended [1].
Surgical indications: Heart failure is the most common complication and the most common cause of death in endocarditis [10]. Infection with Staphylococcus aureus increases the risk of heart failure. In the absence of severe comorbidities, early surgical intervention is usually indicated (Table 2) [1].
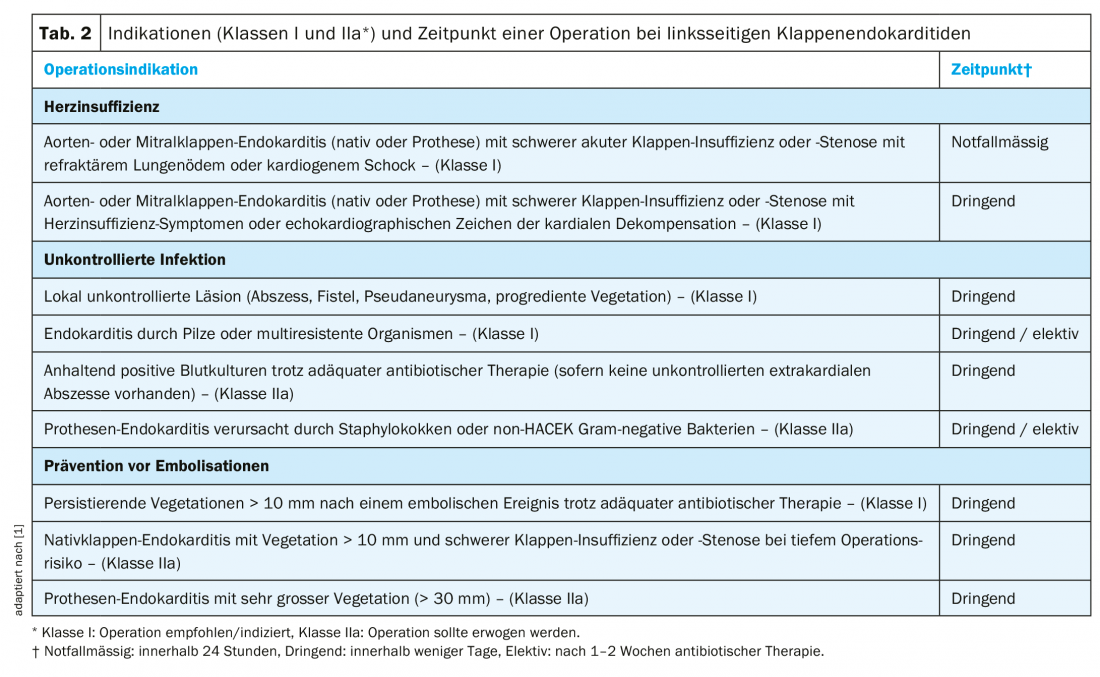
The second most common cause of surgical intervention for endocarditis is an uncontrolled infection (Table 2) [1,10]. This is due to paravalvular extension of infection (abscess, pseudaneurysm, fistula) in the majority of cases. Clinical evidence of a paravalvular complication includes persistent fever or new onset atrioventricular block.
Another feared complication, which may lead to valve surgery, is systemic embolisaton of vegetations (Table 2) [1]. Embolic events occur in 20-50% of patients with endocarditis. However, the risk of new events under adequate antibiotic therapy is lower (6-21%) and strongly decreasing after the 1st week under antibiotics [11,12]. The size and mobility of vegetation are important risk factors for embolization (Table 2) [1,11,12].
Neurologic complications: Symptomatic neurologic complications occur in approximately 15-30% of patients with endocarditis [13]. Cerebral insult is associated with increased mortality. Prompt diagnosis and initiation of adequate antibiotic therapy are of great importance in preventing neurologic complications of endocarditis [12]. After a first ischemic insult, necessary cardiac surgery (Table 2) is not contraindicated and should not be delayed if the neurologic damage is not severe [14]. In the case of a relevant cerebral hemorrhage, on the other hand, min. 1 month to wait for cardiac surgery [15].
Infection of intracardiac electronic devices: Endocarditis associated with a pacemaker or defibrillator is often a diagnostic challenge and is associated with high mortality [1,16]. Treatment usually involves prolonged antibiotic therapy (usually 4-6 weeks) and complete removal of the foreign body [1]. If possible, removal of the electrodes should be done transvenously [17]. When an intracardiac electronic device is implanted, routine antibiotic prophylaxis, usually with an i.v. cephalosporin (started 1 hour before implantation, given for 24-36 hours), is recommended [18].
Take-Home Messages
- Endocarditis prophylaxis is now recommended only for high-risk cardiac patients who are at the highest risk for acquiring and for an unfavorable course of endocarditis
- Echocardiography is the imaging of choice in the diagnosis of endocarditis. Primarily, a TTE is performed; usually followed by a TEE, except in cases of negative TTE and clinically low suspicion
- A cardiac CT, 18F-FDG PET/CT, or SPECT/CT may be helpful as complementary imaging, especially if prosthetic or device-associated endocarditis is suspected
- Indications for surgery include complicated endocarditis with signs of heart failure, uncontrolled infection, or risk of systemic emboli
- Ischemic cerebrovascular insult is not a contraindication and should not delay necessary cardiac surgery in the absence of severe neurologic damage
- In case of device-associated endocarditis, removal of the foreign body and prolonged antibiotic therapy are recommended
Literature:
- Habib G, Lancellotti P, Antunes MJ, et al: 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36: 3075-3128.
- Lockhart B, Brennan MT, Sasser HC, et al: Bacteremia associated with toothbrushing and dental extraction. Circulation 2008; 117: 3118-3125.
- Flückiger U, Jaussi A: Revised Swiss guidelines for endocarditis prophylaxis. Cardiovascular Medicine 2008; 11: 392-400.
- Raoult D, Casalta JP, Richet H, et al: Contribution of systematic serological testing in diagnosis of infective endocarditis. J Clin Micro- biol 2005; 43: 5238-5242.
- Habib G, Badano L, Tribouilloy C, et al: Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010; 11: 202-219.
- Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modi- fications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30: 633-638.
- Hess A, Klein I, Iung B, et al: Brain MRI findings in neurologically asymptomatic patients with infective endocarditis. Am J Neuroradiol 2013; 34: 1579-1584.
- Pizzi MN, Roque A, Fernández-Hidalgo N, et al: Improving the Diagnosis of Infective Endocarditis in Prosthetic Valves and Intracardiac Devices With 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Angiography: Initial Results at an Infective Endocarditis Referral Center. Circulation 2015; 132: 1113-1326.
- Rouzet F, Chequer R, Benali K, et al: Respective performance of 18F-FDG PET and radiolabeled leukocyte scintigraphy for the diagnosis of prosthetic valve endocarditis. J Nucl Med 2014; 55: 1980-1985.
- Tornos P, Iung B, Permanyer-Miralda G, et al: Infective endocarditis in Europe: lessons from the Euro heart survey. Heart 2005; 91: 571-575.
- Vilacosta I, Graupner C, San Roman JA, et al: Risk of embolization after institution of antibiotic therapy for infective endocarditis. J Am Coll Cardiol 2002;39: 1489-1495.
- Dickerman SA, Abrutyn E, Barsic B, et al: The relationship between the initiation of antimicrobial therapy and the incidence of stroke in infective endocarditis: an analysis from the ICE Prospective Cohort Study (ICE- PCS). Am Heart J 2007; 154: 1086-1094.
- Garcia-Cabrera E, Fernandez-Hidalgo N, Almirante B, et al: Neurological complications of infective endocarditis: risk factors, outcome, and impact of cardiac surgery: a multicenter observational study. Circulation 2013; 127: 2272-2284.
- Kang DH, Kim YJ, Kim SH, et al: Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012; 366: 2466-2473.
- Yoshioka D, Sakaguchi T, Yamauchi T, et al: Impact of early surgical treatment on postoperative neurologic outcome for active infective endocarditis complicated by cerebral infarction. Ann Thorac Surg 2012; 94: 489-495.
- Nof E, Epstein LM: Complications of cardiac implants: handling device infections. Eur Heart J 2013; 34: 229-236.
- Sohail MR, Uslan DZ, Khan AH, et al: Infective endocarditis complicating permanent pacemaker and implantable cardioverter-defibrillator infection. Mayo Clin Proc 2008; 83: 46-53.
- Klug D, Balde M, Pavin D, et al: Risk fac- tors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007; 116: 1349-1355.
CARDIOVASC 2019; 18(4): 4-7