The course of psoriatic arthritis has a significant impact on the quality of life of those affected. The German version of the PsAQoL questionnaire is an empirically validated measurement instrument for assessing health-related quality of life in patients with psoriatic arthritis. It is a short procedure that is easy to perform and is suitable for use in clinical practice as well as for research purposes.
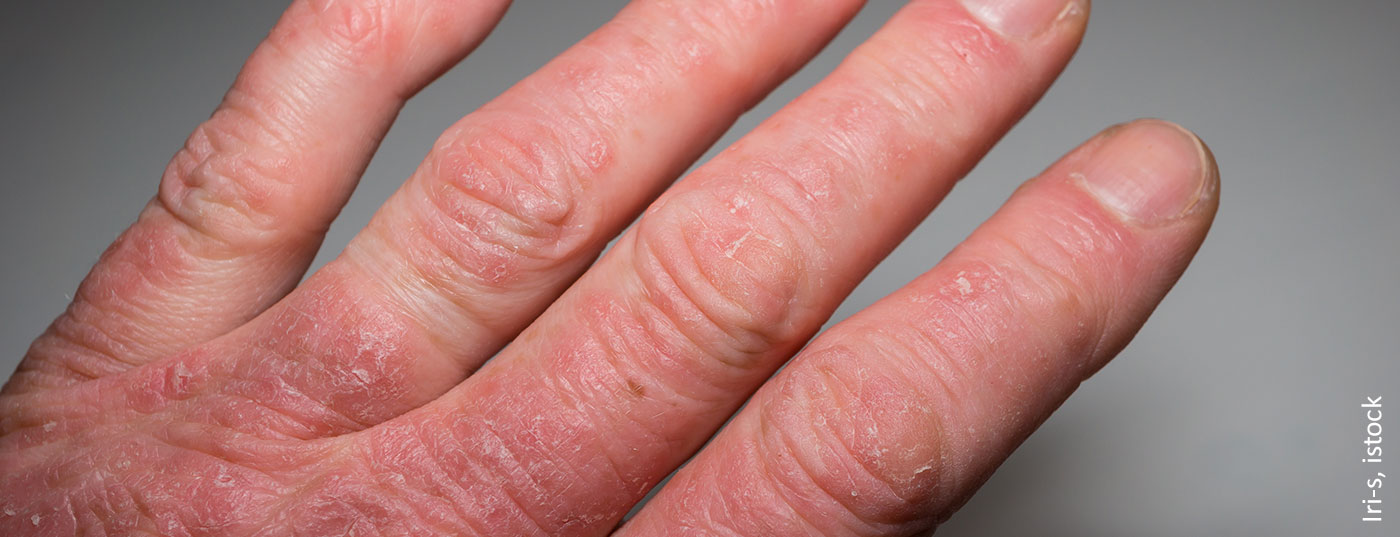
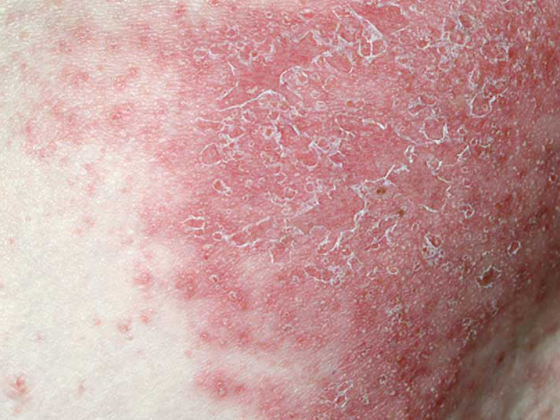
Psoriatic arthritis (PsA) is a chronic inflammatory systemic disease. The clinical symptoms are multifaceted. Pain, stiffness and reduced function of the joints as well as skin manifestations of varying severity are among the core symptoms. If left untreated, it can lead to joint destruction and even to arthritis mutilans and the full-blown form of ankylosing spondylitis [1]. Standardized assessment of health-related quality of life in PsA patients is also becoming increasingly important in the context of indication and therapy evaluation. It is well established that the progression of joint destruction not only results in more severe symptoms, but that health-related quality of life also decreases with increasing functional impairment [2] (box) . Conversely, symptom-relieving and/or disease-modifying therapy also affects health-related quality of life.
Quality of life correlates with severity of PsoA
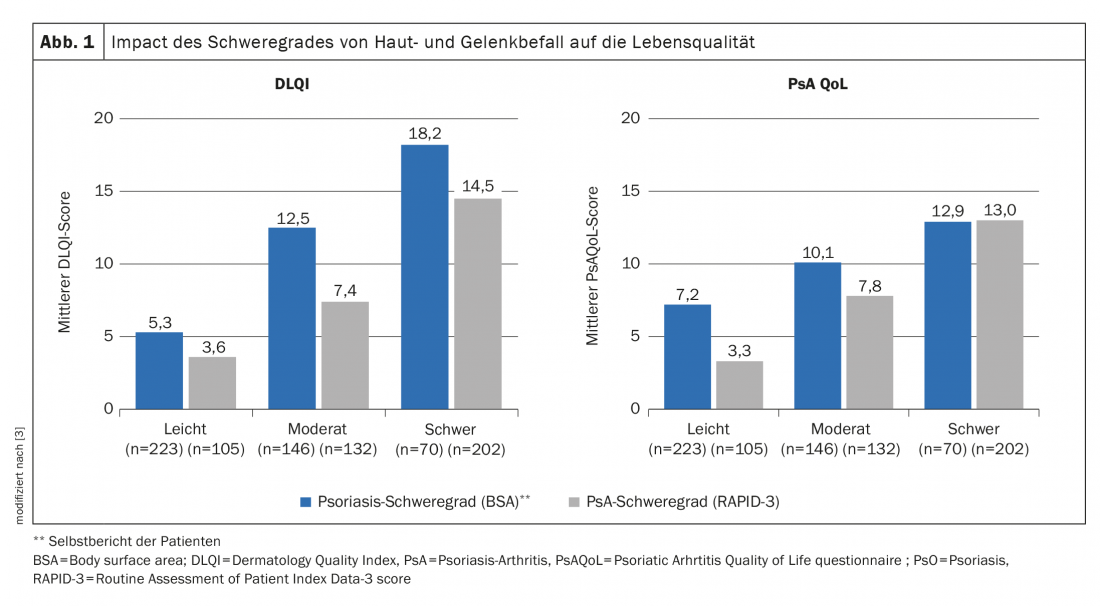
A recent example of empirical evidence that health-related quality of life is associated with symptom severity in PsA patients is data from an international survey study published in 2019 with participants from the United States as well as France and Germany [3]. A total of 439 patients participated in the survey, of whom 23.9% had mild PsA (RAPID3* of 0-2) and 76.1% had moderate-to-severe PsA (RAPID3 of 2,1-10). Plaque psoriasis severity was mild in 51% and moderate to severe in 49%. The study results shown in Figure 1 demonstrate a clear relationship between the severity of joint and skin involvement and quality of life (DLQI, PsAQoL).
* RAPID-3=Routine Assessment of Patient Index Data-3 score
PsAQoL questionnaire: meaningful and user-friendly
The PsAQoL (“Psoriatic Arthritis Quality of Life questionnaire”) is the first validated disease-specific measurement instrument for assessing the health-specific quality of life (HRQoL) of German-speaking patients with PsA (overview 1). It is a rational procedure with a high test methodological quality, as the evaluation of the test quality criteria showed [4]. The original English version of the PsAQoL has been translated into several languages and validated in relation to patient populations in different countries [5]. Because the PsAQoL comprises only 20 questions with a dichotomous response format, completion of the questionnaire can be easily integrated into routine clinical procedures. The tool is easy to use and with an average processing time of less than 5 minutes, it is a time-saving test. For each question, 0 or 1 point is awarded, so that the sum score ranges from 0 (good quality of life) to 20 (poor quality of life). The validation study of the German language version of the PsAQoL was published in the Journal of Rheumatology 2021 [1]. The psychometric properties of the German version of the PsAQoL proved to be good overall, demonstrating the validity and reliability of the questionnaire.

Validation study of the German version proves the good test quality
The PsAQoL questionnaire is based on the principle of needs-based quality of life and was originally developed by McKenna et al. Developed in 2004 in the UK in an English version [6]. The original version was translated into German using the panel method. After a review of the face and content validity of the translated version, the questionnaire was validated in a representative cohort of 126 PsA patients who met CASPAR classification criteria in addition to clinical diagnosis [7]. The study participants recruited in 2011-2012 were inpatients of the Rheumazentrum Ruhrgebiet (D) and outpatients of four rheumatological cooperation practices. Forty-four of the study participants received continuous and 40 received as-needed therapy with nonsteroidal anti-inflammatory drugs (NSAIDs). There were 60 patients on permanent glucocorticoid treatment. The mean prednisolone dose was 3.9 mg ± 6.61 mg. Conventional (csDMARD) basic therapy was given to 97 patients (methotrexate [n=67], leflunomide [n=10], sulfasalazine [n=9], other csDMARDs [n=10], missing data/missing value [n=1]). A bDMARD therapy with a TNFα inhibitor had 18 of the participants. 14 received an additional opioid medication.
|
PsAQoL and DLQI are complementary measurement tools The DLQI primarily measures the impact of skin symptoms on quality of life, but not the full impact of joint involvement. The latter is the focus of the PsAQoL, so parallel use of these two questionnaire tools is useful in psoriasis patients with skin and joint involvement [5]. The recording of PsA-specific functional impairment is a unique feature of the PsAQoL [6]. Among others, the PsAQoL has also been used in some larger RCTs, including a study published in the Lancet by Coates et al. 2015 [8,9]. This was a 48-week clinical trial to investigate the effect of early therapy in PsA patients, demonstrating, as in many other studies, a clear association between symptom severity and quality of life. |
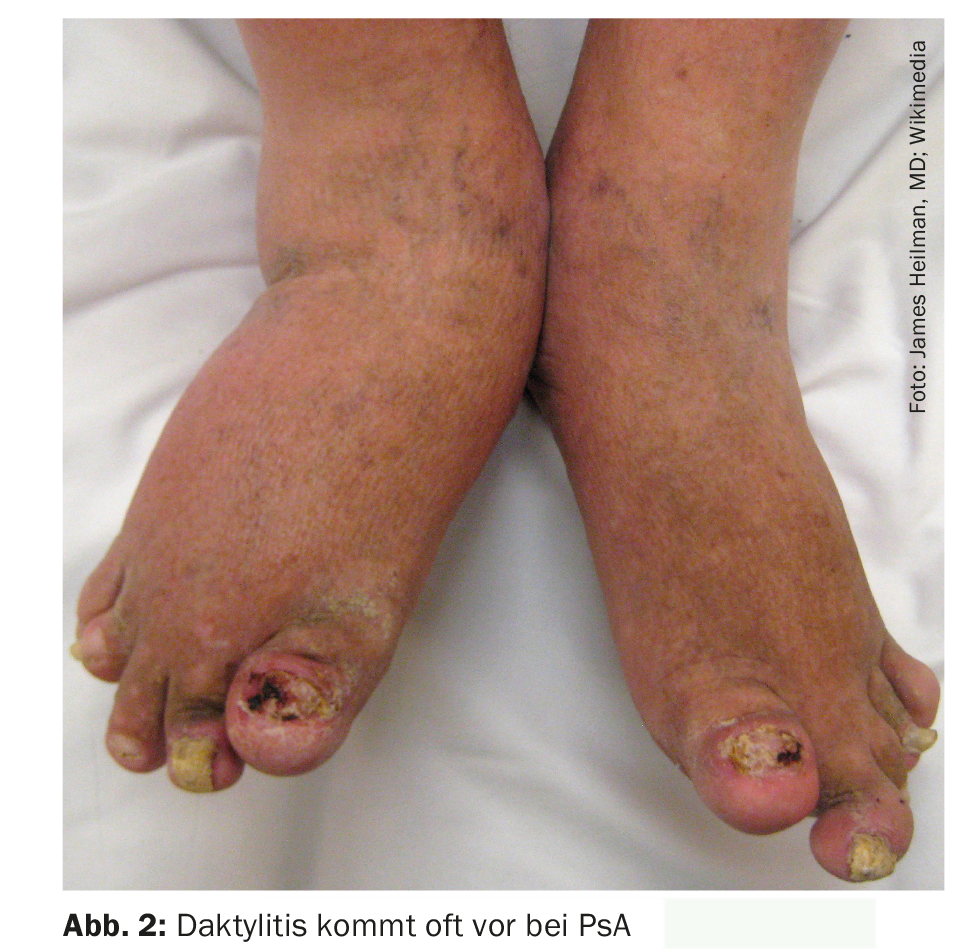
Patients were examined as a baseline visit at week 0 and additionally at week 2 in those with stable disease progression. At baseline (week 0), the PsAQoL had a mean value of 8.7±5.9, with a range of variation from 0 to 20. The survey of test-methodological characteristics yielded the following results:
Construct validity: there was a good correlation of the PsAQoL questionnaire with the following NHP** subdomains at baseline (week 0): energy loss (0.75), pain (0.70), emotional response (0.75), physical mobility (0.70). This reflects the importance of these dimensions to quality of life. PsAQoL and HAQ# (0.65) were moderately correlated, and the same was true for the NHP subdomains of sleep and social isolation.
**NHP=Nottingham Health Profile
# HAQ=Health Assessment Questionnaire
Internal consistency: with a Cronbach’s α coefficient of 0.92 at baseline and 0.95 at week 2, internal consistency for the PsAQoL proved to be high.
Test/retest reliability: questionnaire data from 68 patients with stable disease progression were analyzed to assess retest reliability after 2 weeks. The PsAQoL sum score was 7.7±6.0 at baseline and 7.4±6.5 at the second visit after 2 weeks. A Spearman correlation coefficient of 0.94 was measured, corresponding to very good reliability.
Group validity: a significant difference manifested in the PsAQoL sum score of employed patients compared to the non-employed. The same is true for patients with vs. without use of assistive devices and patients with mild vs. severe pain symptoms.
The robust psychometric test properties of the German version of the PsAQoL are consistent with the original English version as well as with the Swedish, Dutch, Portuguese, and Greek translations.
Literature:
- Kiltz U, et al. Standardized assessment of health-related quality of life in patients with psoriatic arthritis: validation of the German version of the Quality of Life Questionnaire for Patients with Psoriatic Arthritis (PsAQoL). Z Rheumatol 2021; 80(2): 122-131.
- McHugh NJ, Balachrishnan C, Jones SM: Progression of peripheral joint disease in psoriatic arthritis: a 5-yr prospective study. Rheumatology (Oxford) 2003; 42(6): 778-783.
- Merola JF, et al: Patient Perspective on the Burden of Skin and Joint Symptoms of Psoriatic Arthritis: Results of a Multi-National Patient Survey. Rheumatol Ther 2019; 6(1): 33-45.
- Brodszky V, et al: Comparison of the Psoriatic Arthritis Quality of Life (PsAQoL) questionnaire, the functional status (HAQ) and utility (EQ-5D) measures in psoriatic arthritis: results from a cross-sectional survey. Scand J Rheumatol 2010; 39: 303-309.
- Mease P, Strand V, Gladman D: Functional impairment measurement in psoriatic arthritis: Importance and challenges. Semin Arthritis Rheum 2018; 48(3): 436-448.
- McKenna SP, et al: Development of the PsAQoL: a quality of life instrument specific to psoriatic arthritis. Ann Rheum Dis 2004; 63: 162-169.
- Taylor W, et al: CASPAR Study Group Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006; 54: 2665-2673.
- Coates LC, et al: Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet 2015; 386: 2489-2498.
- Gladman D, et al: Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken) 2014; 66: 1085-1092.
- Bloomfield K: A guide to the use of selected health-related quality of life measurement instruments. Berlin Center for Public Health 1996.
DERMATOLOGY PRACTICE 2021; 31(4): 33-34