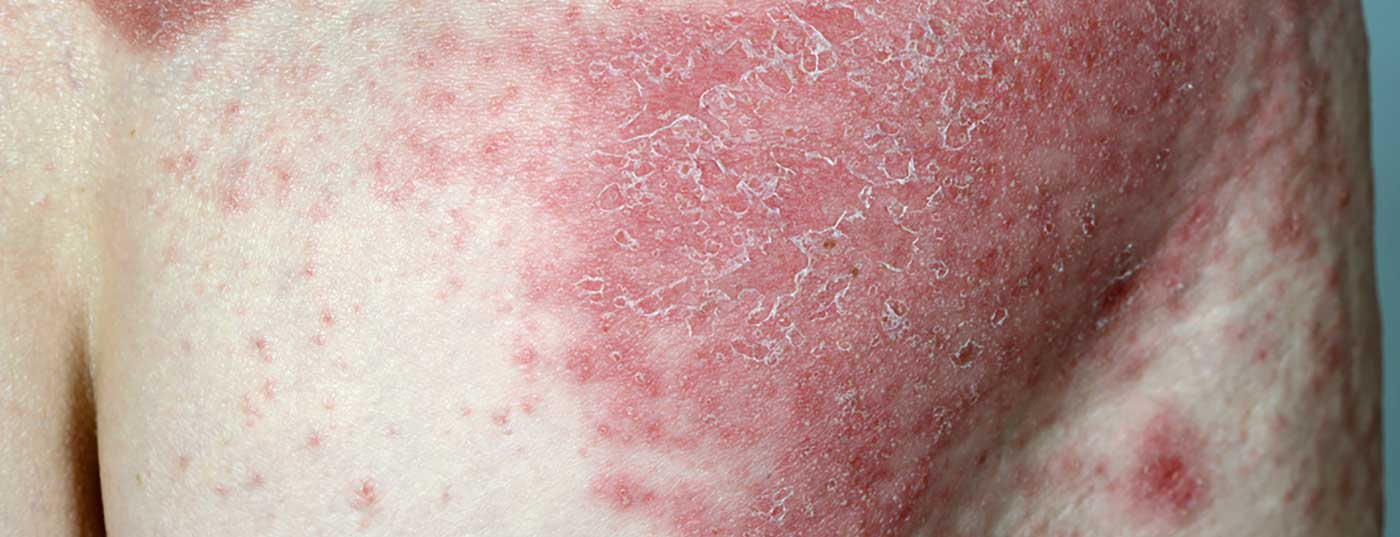
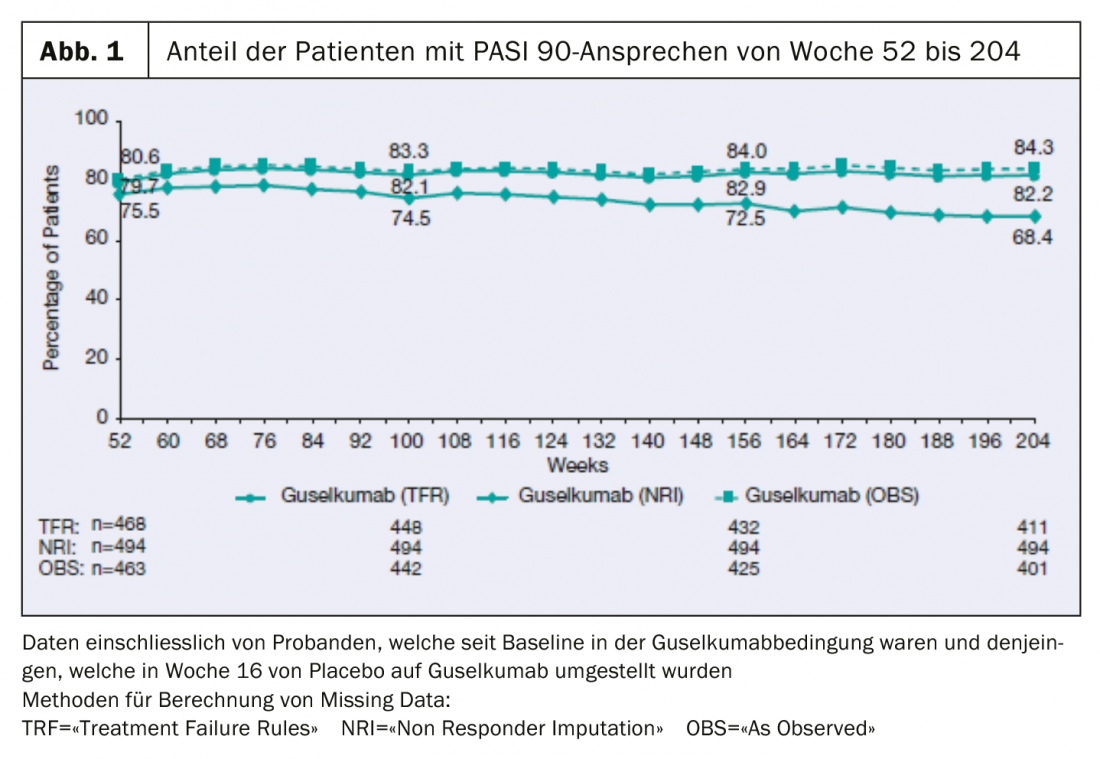
Recently presented long-term data from the VOYAGE 1 Phase III clinical trial demonstrate that 84% of patients continued to achieve PASI-90 at 204 weeks.
The monoclonal antibody guselkumab (TREMFYA®) has been approved in Switzerland since 2018 in adults with moderate-to-severe plaque psoriasis who are eligible for systemic therapy [1]. In the pivotal VOYAGE 1 trial (n=837), guselkumab was shown to be significantly superior to adalimumab and placebo in all primary endpoints (PASI-90 response) and key secondary endpoints (IGA 0/1, PASI-75 response).
Sustained high efficacy and tolerability
Four-year data from the open-label phase of the multicenter, randomized, double-blind VOYAGE 1 study are now available and were presented at the 39th Fall Clinical Dermatology Conference in Las Vegas (USA) [2]: The proportion of patients with PASI 90 remained continuously stable until week 204: 8 out of 10 patients showed a PASI-90 response under guselkumab over this time period (Fig. 1). 82% of patients receiving TREMFYA® (guselkumab) in the combined group of patients initially randomized to the guselkumab or placebo condition with a switch to guselkumab at week 16 achieved at least a 90% improvement in the Psoriasis Area Severity Index (PASI 90) at week 204. In addition, an IGA score of 0 (lesion-free) or 1 (nearly lesion-free) was measurable at this measurement time point.
Guselkumab (TREMFYA®) is the first approved all-human monoclonal antibody that selectively binds to the p19 subunit of interleukin-23 (IL-23) and inhibits its interaction with the IL-23 receptor [1]. This substance thus blocks the release of these inflammatory cytokines, which are involved in the formation of plaques in psoriasis. TREMFYA® was generally well tolerated by patients with psoriasis during clinical development [3–5]. Also, no new safety signals were identified over the open-label period of 4 years [2].
VOYAGE 1 Phase III Follow-up Study
Subjects were randomly assigned at baseline to placebo, guselkumab, or adalimumab conditions. The placebo-controlled treatment phase lasted from week 0 to 16, after which patients in the placebo condition were switched to guselkumab until week 48, and a comparison was made with the active substance adalimumab (weeks 0-48). Study participants were randomized to guselkumab at week 0, and those who switched from placebo to guselkumab at week 16 received guselkumab at week 48, 8 weeks apart. From week 52, all participants received guselkumab. Efficacy was assessed using the Psoriasis Area and Severity Index (PASI75/90/100) and IGA scores (0=lesion-free, 1=almost lesion-free). Missing data were calculated using the following methods: NRI (“Non-Responder Imputation Rules”), TFR (“Treatment Failure”) and OBS (“As Observed”).
Source: Janssen-Cilag AG
Literature:
- Technical Information Tremfya®, 02/2019, www.swissmedicinfo.ch, last accessed 24 Oct 2019.
- Griffiths CE, et al: Maintenance of response with up to 4 years of continuous guselkumab treatment: results from the VOYAGE 1 phase 3 trial. Falls Clinical Dermatology Conference. Oct, 2019; Las Vegas, USA.
- Blauvelt A, et al: Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol 2017; 76(3): 405-417.
- Reich K, et al: Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76(3): 418-431.
- Langley R, et al: Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol 2018; 178(1): 114-123.
DERMATOLOGIE PRAXIS 2019; 29(6): 31 (published 8/12/19, ahead of print).