By bringing together research fields that were previously pursued independently, such as neuroscience, metabolism and diabetology, and psychology, knowledge is also growing for diseases as complex as human obesity. Dr. Olivia M Farr and Prof. Christos S Mantzoros of Harvard Medical School are working hard to elucidate the communication pathways between the brain and adipose tissue. In Munich at the 52nd European Diabetology Congress EASD, they showed what is already known.
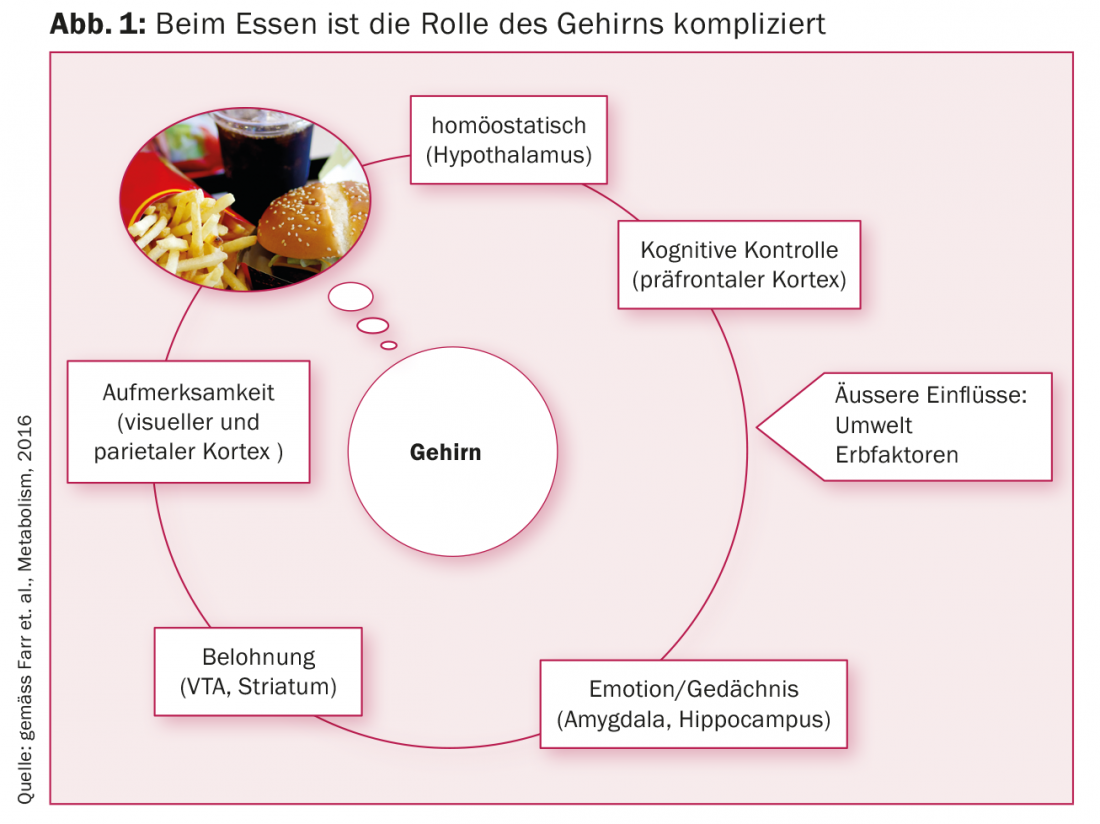
The brain constantly receives signals from all organs involved in metabolism. It processes the information and in turn gives commands to all metabolically active cells with the aim of establishing and maintaining homeostasis. Which switching centers in the brain control our metabolism to optimally adjust it to our environment is only cryptically known so far. “The brain integrates information from inside and outside, we want to know where exactly the switching points are,” explained Prof. Christos S Mantzoros of Harvard Medical School at the European Diabetes Congress in Munich (Fig. 1).
We eat because what is there
One explanation for the current obesity epidemic, he says, is a very simple one, namely that we rarely eat hungrily, but because it simply feels good, tastes good, and the supply is so great. Why some people remain slim while others become obese is the big black box. Key players, according to Dr. Farr and Prof. Mantzoros, are adipocytes and specific brain centers. “The reward effect of good satiators is comparable to cocaine,” Dr. Farr pointed out (Fig. 2).

Abdominal adipose tissue is not only responsible for the storage of triglycerides. It also regulates metabolic and inflammatory processes in multiple ways that go far beyond storing energy in the form of fat. Known effects are on the cardiovascular system, insulin sensitivity, and insulin secretion. If the signal exchange between the central nervous system and adipose tissue gets out of control due to internal and/or external influences, it drifts towards malfunction and a vicious circle begins [1].
Leptin and brain
Prof. Mantzoros and his research group are particularly interested in the messengers leptin, irisin and adiponectin from adipocytes, but also focus on ghrelin, insulin and PYY. Using functional magnetic resonance imaging (fMRI), they are studying animals and humans at Harvard to determine which regions in the brain “light up” when appetizing foods are shown, for example. Dr. Olivia M Farr emphasized that the complex brain of humans is difficult to compare with that of other mammals. A fundamental problem with the current obesity epidemic from a neuropsychological perspective is that fatty high-calorie foods in particular are perceived as highly desirable and are available almost everywhere, Dr. Farr explained.
“Where does desire arise, how are leptin receptors distributed, what happens when we give recombinant leptin?” are current questions surrounding leptin. The basic function of this prohormone is to signal to the brain when energy stores are full enough. If the hormone cannot be produced because of a change in the genetic material, the brain does not receive a saturation signal. As a result, food is ingested without restraint, and extreme obesity develops. Recombinant leptin cannot be used to treat obesity but is an option in HIV-related lipodystrophy. This is the first clinically relevant result from Harvard [2].
Emotional eaters
In their search for leptin receptors and how to influence them, the Harvard researchers studied lorcaserin, among other things. This serotonin-5-hydroxytryptamine 2c receptor agonist could effectively treat obese patients who eat primarily for emotional reasons and/or as a reward, Prof. Mantzoros elaborated. A feeling of satiety is created and the calorie intake is slowed down. The fMRI showed decreased activity in the parietal and visual cortex and in the insula and amygdala. Those areas that respond strongly to food offers in emotional eaters [3].

The brain regions responsible for reward and involved in eating behavior also show a divergent structure compared to normal weight individuals. Overweight and obesity are also associated with changes in neurotransmitters in the brain. Specifically, the opioid system is different than in normal weight individuals. In this respect, there is speculation as to whether overweight and obesity is comparable to addictive disorders, to excessive cravings and lack of self-control, and to the need for ever-larger amounts of food. This has been demonstrated by Finnish scientists and published in the “Journal of Neuroscience” [4]. The object of research will now be to determine whether these changes result from uncontrolled food intake or whether they are due to a genetic predisposition.
Glucagon and brain
Why do diabetes patients taking the glucagon-like peptide analog liraglutide lose weight? This question was also investigated at Harvard. Liraglutide activates the GLP-1 receptor, which is involved in the regulation of appetite and activates centers in the brain that lead to a feeling of satiety. As a result, those treated eat less and lose weight. In addition, liraglutide stimulates insulin secretion and decreases glucagon secretion, resulting in a decrease in postprandial and fasting blood glucose [5].
Prof. Matthias H. Tschöp and his research group at the Helmholtz Institute for Diabetes and Obesity in Munich have also set out to analyze the role of the brain in blood glucose balance in greater detail. Their conclusion, published in the scientific journal Nature, is that normal blood glucose regulation depends on a partnership between the insulin-producing cells of the pancreas on the one hand and neuronal circuits in the hypothalamus and other brain regions on the other [6]. According to the new model, leptin activates the regulatory system in the brain, which then boosts glucose utilization – including via other hormones that are independent of insulin. These make a similar contribution to sugar metabolism as insulin. For type 2 diabetes to break out, both systems must be disrupted at the same time.
Scientists have also shown that astrocytes respond to leptin. This is an important factor for the feeling of satiety. Since both leptin and insulin have been shown to affect astrocytes, the researchers propose to develop a new model, which would include astrocytes as regulators of metabolism and hunger in addition to neurons. From the more detailed picture they will then have, they hope to gain new perspectives for drug development.
Source: 52nd EASD Annual Meeting, 12-16 September 2016, Munich, Germany
Literature:
- Farr OM, et al: Central nervous system regulation of eating: Insights from human brain imaging. Metabolism. 2016 May; 65(5): 699-713.
- Farr OM, et al: Leptin applications in 2015: what have we learned about leptin and obesity? Curr Opin Endocrinol Diabetes Obes. 2015 Oct; 22(5): 353-9.
- Farr OM, et al: Lorcaserin Administration Decreases Activation of Brain Centers in Response to Food Cues and These Emotion- and Salience-Related Changes Correlate With Weight Loss Effects: A 4-Week-Long Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabetes. 2016 Oct; 65(10): 2943-53.
- Karlsson H K, et al: Obesity Is Associated with Decreased μ-Opioid But Unaltered Dopamine D2 Receptor Availability in the Brain. The Journal of Neuroscience, 4 March 2015, 35(9): 3959-3965.
- Farr OM, et al: GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia. 2016 May; 59(5): 954-65.
- Caceres, C. et al: (2016): Astrocytic insulin signaling couples brain glucose uptake with nutrient availability, Cell, DOI: 10.1016/j.cell.2016.07.028 www.cell.com/cell/fulltext/S0092-8674(16)30974-6.
CARDIOVASC 2016; 15(6): 33-36