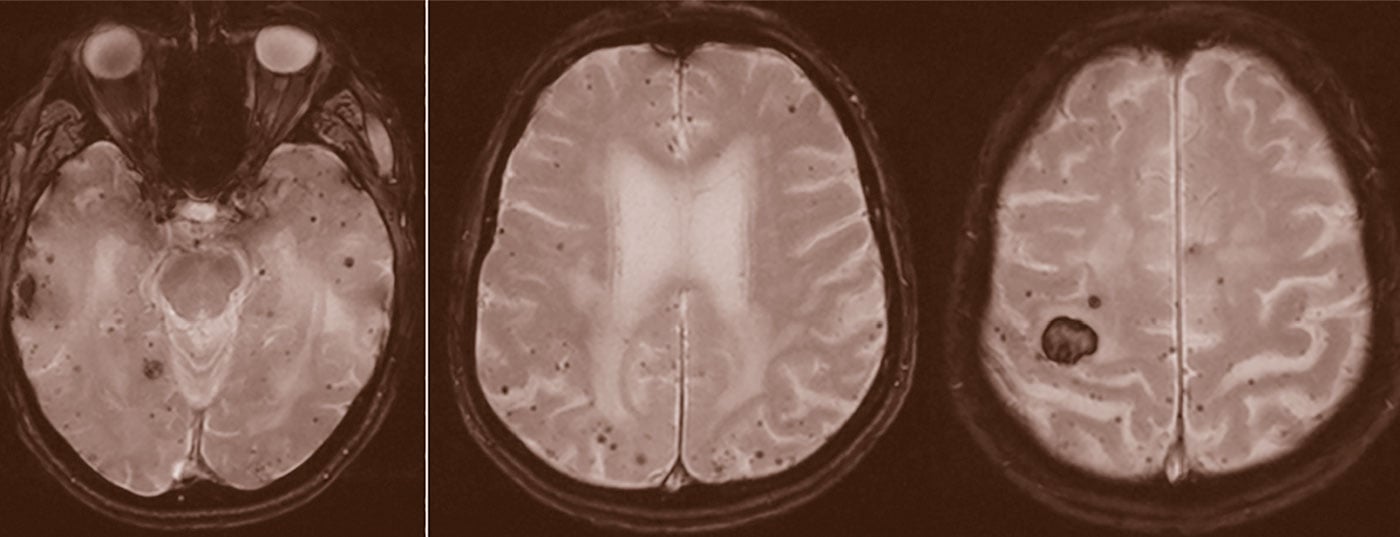
Stroke is a common disease. It most often leads to permanent disability in adulthood. Therefore, research projects are being conducted to improve diagnostics and therapy.
Stroke is a common disease. Globally, between 1990 and 2010, the number of patients with ischemic and hemorrhagic stroke increased by 37% and 47%, respectively. Since the absolute number of deaths also increased as a result and stroke is the disease that most frequently leads to permanent disability in adulthood, numerous research projects are being conducted that are primarily aimed at improving diagnosis, acute therapy and secondary prevention. In the following, an overview of current study results and the consequences for clinical routine will be outlined. Since ischemic strokes (IS) account for 85-90% of all strokes and the most important results were obtained in patients with IS, the focus of this work will be on them.
Acute therapy – “time is brain”!
Recanalizing therapies represent the basis and basic requirement of successful acute treatment of IS. This currently includes intravenous thrombolytic treatment with recombinant tissue-type plasminogen activator (“rtPA”) [1] and mechanical thrombectomy, usually performed with stent retrievers [2]. Common to both treatment options is the fact that the therapy is time-critical, which is summed up by the well-known slogan “time is brain”. It is estimated that in acute IS, almost two million neurons per minute are irreversibly destroyed. Pathophysiologically relevant is the penumbra concept, according to which a so-called infarct core is irreversibly lost very soon after symptom onset, the penumbra surrounding the infarct core, but at least briefly supplied by collaterals and thus potentially salvageable. At a certain point, the potential harm of the recanalizing measure exceeds the expected benefit, so that the therapy should then not be performed. Whereas only a few years ago attempts were made to establish generally binding time windows (intravenous thrombolysis: 4.5 hours; thrombectomy: 6 hours), it is becoming increasingly clear that there are large interindividual differences in the dynamics of infarct progression, which presumably depend significantly on the collateral status of the respective patient. Accordingly, the therapy, i.e. the assessment of the time window, of the recanalizing treatment must also be individually adapted. In the following, various examples will explain the current study situation in this field.
Thrombectomy sometimes successful even hours after symptom onset
The DAWN study [3] examined the outcomes of thrombectomies within a six- to twenty-four-hour time window (“last seen well”). Patients with occlusion of the intracranial internal carotid artery (ACI) or the M1 segment of the middle cerebral artery (ACM) demonstrated by CT angiography (CTA) or MR angiography (MRA) were included if a mismatch was found between infarct volume and severity of clinical symptoms. Additional inclusion criteria included a premorbid modified Rankin Scale (mRS) score of 0-1. Patients were randomized to the thrombectomy group (n=107) and the control group (n=99). The median National Institutes of Health Stroke Scale (NIHSS) score was 17 in both groups, and the median infarct volume was 7.6 ml in the thrombectomy group and 8.9 ml in the control group. At 90 days, 49% from the thrombectomy group were functionally independent (mRS 0-2), compared with only 13% in the control group. Already after 24 hours, there was a difference in median infarct volume (8 ml in the thrombectomy group vs. 22 ml in the control group). Adverse endpoints did not differ in the two groups.
Another study that investigated the procedure of thrombectomy within an extended time window is the DEFUSE-3 trial [4]. A time window of 6-16 hours was examined here (thrombectomy group: n=92; control group: n=90), with initial infarct volume of maximum 70 ml and a ratio of infarct volume to threatened area of at least 1.8 with a penumbra of at least 15 ml. There had to be occlusion of the ACI or proximal ACM confirmed with CTA or MRA. The primary endpoint chosen was the score of the mRS at 90 days. In addition, infarct volume at 24 hours, infarct volume gain, reperfusion, and reopening of the occluded artery were evaluated. At 90 days, 45% of the thrombectomy group and 17% of the control group were functionally independent. In the thrombectomy group, the median increase in infarct volume was 23 ml, whereas in the control group it was 33 ml.
Both studies indicate that in selected patients, thrombectomy can be performed with clinical success even many hours after symptom onset. It is imperative that this be considered in clinical routine.
24/7 emergency MRI in case of unclear time window
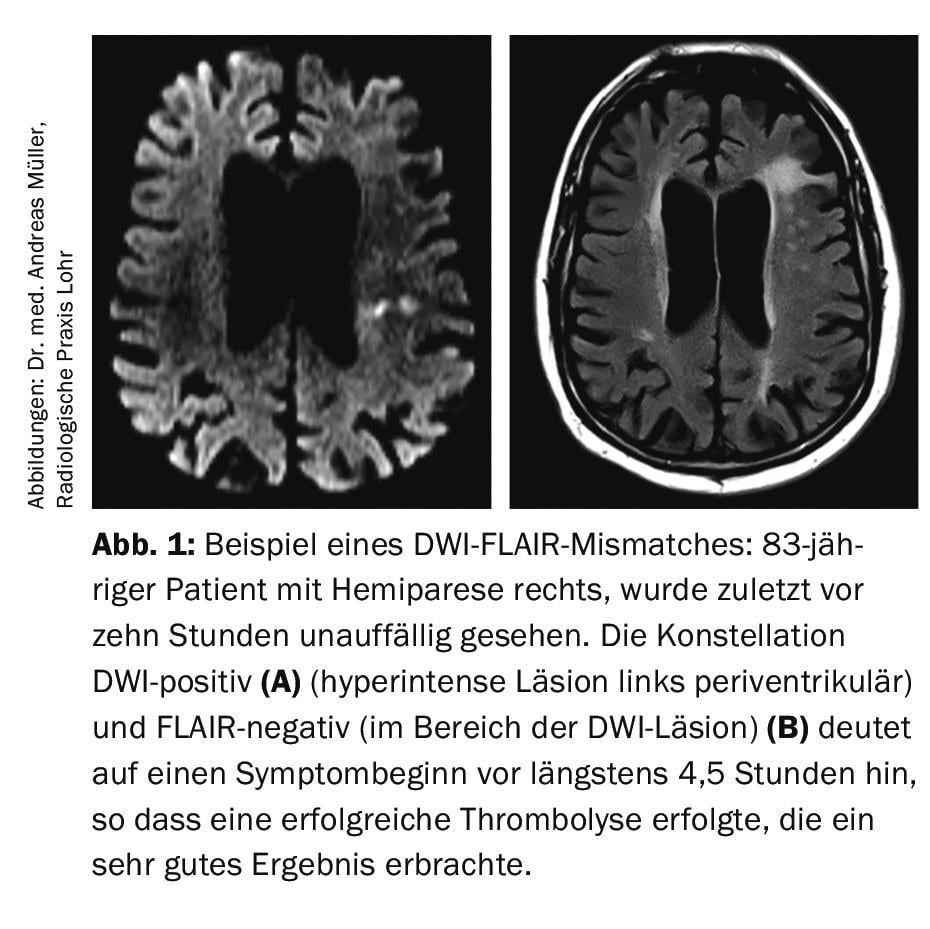
It is known that in up to 30% of all IS, the time window (i.e., symptom onset) is unknown, so that formally there is a contraindication to intravenous thrombolysis according to approval criteria of rtPA. With the aim of treating these patients with intravenous thrombolysis after all, the Hamburg research group led by Prof. Dr. med. Götz Thomalla evaluated the so-called DWI-FLAIR mismatch imaging (DWI=”diffusion weighted imaging”; FLAIR=” fluid-attenuated inversion recovery”). The DWI sequence shows cytotoxic edema, which usually develops very early after the onset of ischemic stroke. In contrast, the FLAIR sequence indicates vasogenic edema, which usually does not develop until 4.5 hours after infarct onset. It follows that if a patient presents with a DWI-positive but no FLAIR signal abnormality, it is relatively likely that the time of symptom onset is within 4.5 hours before the MRI is performed (Fig.1). In the multicenter randomized and placebo-controlled wake-up study, patients with IS (DWI-positive, FLAIR-negative) were included and randomized 1:1 between rtPA 0.9 mg/kg body weight or placebo [5]. The study was stopped early because in an interim analysis, the primary endpoint (mRS at 90 days 0-1 points) was met significantly more often in the verum group (53.3%) than in the placebo group (41.8%). The rate of intracranial hemorrhage was nonsignificantly increased in the verum group, 2.0%, compared with the placebo group, 0.4%. Overall, the study was hailed as a great success. It led to the need to keep an option for emergency MRI available around the clock so as not to miss a thrombolytic opportunity if the time window was unclear.
Critically thinking neurologists have long wondered whether, in an individual patient, it might not also be conceivable to a) despite a time window <4.5 hours not to perform thrombolysis (because e.g. the collaterals are very poor and already after a short time a large infarct core or a small/no penumbra is present) respectively b) despite a time window >4.5 hours to perform thrombolysis in cases where there is a relatively small infarct core and a large penumbra.
While the constellation a) has hardly been investigated and is also likely to occur much less frequently, is constellation (b) frequently encountered in routine clinical practice. Recent studies are available that investigated intravenous thrombolysis up to 9 hours after the onset of ischemic stroke. The multicenter randomized and placebo-controlled EXTEND trial included 225 patients with IS and hypoperfused brain areas including potentially salvageable penumbra in the time window 4.5 to 9 hours after symptom onset and randomized 1:1 to the 0.9 mg/kg body weight or placebo groups [6]. After publication of the positive wake-up study [5], the EXTEND study was also terminated prematurely. The primary endpoint (mRS at 90 days 0-1 points) was achieved in 35.4% of patients in the verum group and in 29.5% of patients in the placebo group (p=0.04). Intracerebral hemorrhages were diagnosed in 6.2% of cases in the verum group and 0.9% of cases in the placebo group (p=0.05).
Thus, in summary, intravenous thrombolysis 4.5 to 9 hours after symptom onset in a selected patient population leads to a better clinical outcome at 90 days despite an increased rate of cerebral hemorrhage. This statement is supported by a recent meta-analysis [7]. However, an immediate transfer of these results into general clinical routine seems to be too early. Ultimately, however, it can be assumed that in the coming years there will be an increasing individualization of acute therapy of ischemic stroke based on multimodal imaging.
Tenecteplase as an alternative for alteplase?
For thrombolysis, currently only alteplase (0.9 mg/kg body weight, max. 90 mg) approved. Alternative compounds continue to be explored with the goal of providing therapy with greater safety and efficacy. Tenecteplase could be a promising alternative.
Tenecteplase has a higher affinity for fibrin as well as a longer half-life. This would allow application via a bolus as opposed to the one-hour infusion with alteplase. In the NOR-TEST study [8], 1100 patients were treated, 549 with tenecteplase (0.4 mg/kg) and 551 with alteplase (0.9 mg/kg). The endpoint of an mRS of 0-1 at three months was met by 64% of patients in the tenecteplase group and 63% in the control group. No superiority of tenecteplase over alteplase was found here, nor did the safety profile show any significant differences.
In contrast, the EXTEND-IA TNK trial [9] investigated so-called bridging lysis before thrombectomy using tenecteplase (n=101, dose 0.25 mg/kg) versus alteplase (n=101). Patients were in the 4.5-hour time window and were scheduled for thrombectomy for proven occlusion of the ACI, ACM, or basilar artery. The primary end point was reperfusion of at least 50% or absence of interventricular thrombus. This endpoint was achieved in 22% of patients in the tenecteplase group and 10% in the control group. Functional outcome at 90 days was also improved in the tenecteplase group (median mRS 2 vs. 3), with no clustering of adverse effects. Further studies, TASTE and ATTEST, did not show superiority of tenecteplase over alteplase, but pooling of patients showed an advantage for those patients who had complete vessel occlusion with complete recanalization at 24 hours in 71% of the tenecteplase group versus 43% in the control group [10].
Further studies are underway. The results to date suggest at least noninferiority of tenecteplase with simpler mode of application, which could be a relevant advantage especially in the case of secondary transport before thrombectomy. The next few years will show whether the very promising thrombolysis alternative with tenecteplase will actually enter clinical routine.
Secondary prevention by means of oral anticoagulation or atrial appendage closure.
Any currently approved antithrombotic therapy for secondary prevention of IS increases the likelihood of bleeding complications. In the case of atrial fibrillation, drug therapy consists of oral anticoagulation. Vitamin K antagonists (e.g., phenprocoumon, target INR 2-3) or non-vitamin K-dependent oral anticoagulants (NOAKs, anti-Xa inhibitors, or the direct thrombin inhibitor dabigatran), which approximately halve the risk of cerebral hemorrhage compared with vitamin K antagonists, are possible for this purpose.
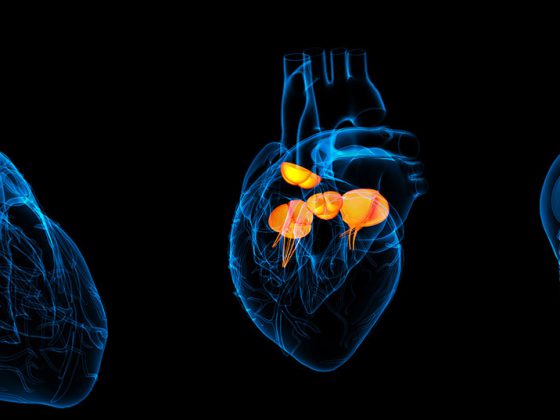
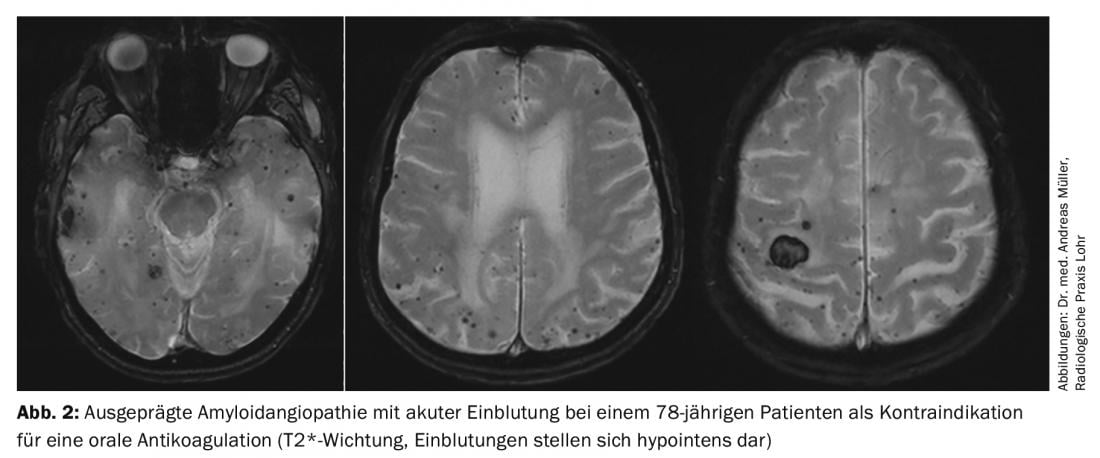
Nevertheless, there are patients who cannot be treated with oral anticoagulants due to an increased risk of bleeding. For these, interventional atrial appendage closure is considered in principle. It is believed that the left atrium is where 90% of all cardiac thrombi are formed. Various devices have been developed for atrial appendage closure, with the current data being best for the so-called Watchman® device. Recently, 5-year data on the PREVAIL and PROTECT AF trial were published, showing a lower risk of bleeding and mortality compared with warfarin [11]. In summary, interventional closure of the left atrial appendage using the Watchman® device may offer advantages, particularly in patients at high risk of bleeding (high HASBLED score) or contraindicated to oral anticoagulation, as the risk of bleeding (especially cerebral hemorrhage) is significantly reduced (Fig. 2) . Critically, we note: (a) lack of long-term data, (b) periinterventional complications, c) Possibility of cardiac thrombi being formed outside the atrial ear, (d) Need for temporary dual platelet function inhibition. The comparison between NOAKs and atrial appendage closure, for which no studies have been published, is still under discussion. It is conceivable that the advantage of atrial appendage closure in terms of reduction of bleeding complications is not present with anticoagulation with NOAKs.
Under 60-year-olds with ESUS and PFO benefit from PFO closure plus TFH
For another cardiac intervention, the study situation changed significantly in 2017. Whereas until then patients with IS and persistent foramen ovale (PFO) as the putative cause of stroke were treated only with a platelet function inhibitor (TFH), several papers demonstrated that in selected patients up to 60 years of age with cryptogenic stroke and PFO with at least moderate right-to-left shunt, interventional PFO closure plus TFH is superior to TFH alone [12–14]. Oral anticoagulation explicitly offers no benefits. The age limit of 60 years is based on the presumption that older patients are more likely to have an alternative cause of stroke than younger patients (namely, primarily atrial fibrillation). Ultimately, however, the line is arbitrarily drawn and it remains to be seen whether patients over 60 years of age may not benefit from PFO closure.
Since the term “cryptogenic stroke” is not well defined, the term ESUS (“embolic stroke of undetermined source”) was introduced in 2014 [15]. Based on this precise definition, it was investigated whether patients with ESUS (i.e., with suspected but not proven AF) are better protected against recurrence of IS by NOAK than by ASA. The NAVIGATE-ESUS trial, which compared rivaroxaban with ASA, had to be terminated prematurely after 3609 patients because no benefit was expected in the intervention group [16]. The RE-SPECT-ESUS trial similarly confirmed that oral anticoagulation vs TFH with ASA does not confer a benefit to the overall ESUS group [17]. The latter study enrolled 5390 patients from 564 study centers. They received either ASA 100 mg or dabigatran at 110 mg or 150 mg twice daily, respectively. The primary endpoint was recurrent IS. After a median of 19 months, 4.1% of patients had a second event per year in the dabigatran arm, compared with 4.8% per year in the ASA arm of the study. The relative risk reduction of 15% did not reach statistical significance (p=0.10). Major bleeding occurred in 1.7% (dabigatran) and 1.4% (ASA) of patients per year. There was a significant difference to the disadvantage of dabigatran in clinically relevant non-severe bleeding (1.6 vs. 0.9% per year). The ATTICUS and ARCADIA trials comparing apixaban with ASA are pending. Regardless, there is increasing evidence that in the case of ESUS, oral anticoagulation with NOAK is superior to TFH in selected patients (eg, with PFO [18] or enlargement of the left atrium [19]). However, further studies are needed to reproduce these results.
Double TFH may prevent recurrences
It has been clear for many years that sustained dual TFH (usually with ASA and clopidogrel) is unfavorable in the risk-benefit balance. In certain constellations, however, a temporarily limited double TFH may be useful and necessary. Known causes include symptomatic intracranial stenoses [20] or stent placements in brain-supplying arteries. In contrast, the finding that patients with TIA and high risk of recurrence (ABCD2 score ≥4) or mild IS (“minor stroke,” NIHSS ≤3) are better protected from early ischemic recurrence events by dual TFH with ASA and clopidogrel for a duration of 10-21 days than by monotherapy with ASA [21,22] is new. The risk of bleeding is increased. However, the authors of the POINT trial estimate that double TFH prevents three times more recurrent IS than major bleeding occurs [22]. It is expected that a recommendation will soon be included in the guidelines of the German Society of Neurology (DGN). A positive statement regarding the procedure has already been published on the DGN homepage.
Outlook
The listed therapeutic achievements of recent years reflect intensive research activity and a strictly evidence-based approach. It is to be hoped and also assumed that this development will continue. In all likelihood, stroke treatment will become more complicated as individual patients (groups) who respond particularly well or not well to a particular therapeutic option are increasingly identified. The individualization of medicine is progressing.
Literature:
- Hacke W, et al: Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359(13): 1317-1329.
- Berkhemer OA, et al: A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372(1): 11-20.
- Nogueira RG, et al: Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11-21.
- Albers GW, et al: Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med 2018; 378(8): 708-718.
- Thomalla G, et al: MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N Engl J Med 2018; 379(7): 611-622.
- Ma H, et al: Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N Engl J Med 2019; 380(19): 1795-1803.
- Campbell BCV, et al: Extending thrombolysis to 4.5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet 2019; 394(10193): 139-147.
- Logallo N, et al: Tenecteplase versus alteplase for management of acute ischaemic stroke (nor-test): a phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol 2017; 16: 781-788.
- Campbell BCV, et al: Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med 2018; 378(17): 1573-1582.
- Bivard A, et al: Tenecteplase in ischemic stroke offers improved recanalization. Neurology 2017; 89(1): 62-67.
- Reddy VY, et al: 5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol 2017; 70(24): 2964-2975.
- Søndergaard L, et al: Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med 2017; 377(11): 1033-1042.
- Mas JL, et al: Patent foramen ovale closure or anticoagulation vs antiplatelets after stroke. N Engl J Med 2017; 377(11): 1011-1021.
- Saver JL, et al: Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med 2017; 377(11): 1022-1032.
- Hart RG, et al: Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014; 13(4): 429-438.
- Hart RG, et al: Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. N Engl J Med 2018; 378(23): 2191-2201.
- Diener HC, et al: Dabigatran for Prevention of Stroke after Embolic Stroke of Undetermined Source. N Engl J Med 2019; 380(20): 1906-1917.
- Kasner SE, et al: Rivaroxaban or aspirin for patent foramen ovale and embolic stroke of undetermined source: a prespecified subgroup analysis from the NAVIGATE ESUS trial. Lancet Neurol 2018; 17(12): 1053-1060.
- Healey JS, et al: Recurrent Stroke With Rivaroxaban Compared With Aspirin According to Predictors of Atrial Fibrillation: Secondary Analysis of the NAVIGATE ESUS Randomized Clinical Trial. JAMA Neurol 2019, in press.
- Chimowitz MI, et al: Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011; 365(11): 993-1003.
- Prasad K, et al: Dual antiplatelet therapy with aspirin and clopidogrel for acute high risk transient ischaemic attack and minor ischaemic stroke: a clinical practice guideline. BMJ 2018; 363: k5130.
- Johnston SC, et al: Clopidogrel and aspirin in Acute Ischemic Stroke and High-Risk TIA. N Engl J Med 2018; 379(3): 215-225.
InFo NEUROLOGY & PSYCHIATRY 2019; 17(5): 5-9