Current European guidelines for the management of heart failure recommend the use of risk scores. Among these, the MECKI score (Metabolic Exercise Test Data Combined with Cardiac and Kidney Indexes) has proven to be one of the most accurate. Nevertheless, risk scores are poorly used in clinical practice, in part because there is a lack of strong evidence to support their external validation in diverse populations.
Heart failure (HF) is a major public health problem with a current prevalence of over 23 million people worldwide [2]. Despite major advances in drug and device therapy, the prognosis remains poor. In the Olmsted County cohort for all types of HF patients, the 1-year and 5-year mortality rates between 2000 and 2010 were 20% and 53%, respectively [3]. In a study combining cohorts from the Framingham Heart Study and the Cardiovascular Health Study, a mortality rate of 67% was found within five years of diagnosis [4].
As a result, the number of HF patients reaching end-stage requiring advanced mechanical circulatory support and/or heart transplantation (HTx) is increasing, which is at odds with the limited number of available organs and a 1-year mortality rate of 20% while on the waiting list [5]. Health authorities have developed prioritization strategies aimed at reducing the growing discrepancy between the number of organs available and potential recipients. Deciding which candidates are appropriate for HTx will be even more frequent and difficult for the physician involved with HF. This is especially true for non-inotropic outpatients, as avoiding delays in admitting higher-risk patients must be carefully weighed against deferring less ill patients. Therefore, it is important to correctly determine the prognosis of HF patients.
Over the past three decades, a number of scores have been developed that combine multiple variables to assist clinicians in assessing patient prognosis. In 2013, the MECKI score was proposed by an Italian working group to assess the risk of cardiovascular (CV) mortality and urgent HTx. It is based on six variables: Hemoglobin (Hb), sodium (Na+), renal function using the Modification of Diet in Renal Disease (MDRD) equation , left ventricular ejection fraction (LVEF) by echocardiography, percent of predicted peak oxygen consumption (ppVO2), and slope of minute ventilation and carbon dioxide production (VE/VCO2). The above variables are recognized prognostic markers in HF, reflecting the complexity and multiorgan involvement of this syndrome: They were determined after multivariate analyses in large populations [6,7].
In recent comparisons, the MECKI score showed good discriminatory power, which is higher than that of other commonly used scores such as Heart Failure Survival Score (HFSS), Seattle Heart Failure Model (SHFM), and Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) [8,9]. Another study [10] showed that the MECKI score can also be used with the advantage that it is very well calibrated at 1-year intervals, which makes it possible to avoid the pitfalls of underestimating or overestimating risk. Nevertheless, few of these risk scores are used in clinical practice, in part because of a lack of strong evidence to support their external validation in diverse populations [11]. Therefore, the current study was designed as an external validation test of the MECKI score in an international multicenter setting [1].
Study population
A total of 1042 patients from eight international centers (seven European and one Asian) were enrolled in the study. Inclusion criteria were (i) previous or current HF symptoms, (ii) History of reduced left ventricular systolic dysfunction (LVEF ≤45%), (iii) Stable clinical condition with no change in medication in the past three months, (iv) no planned major cardiovascular treatment or intervention; and (v) Performance of a maximal cardiopulmonary exercise test (CPET), regardless of respiratory exchange ratio, achieved with a ramp loading protocol (steps no longer than one minute) on a treadmill or cycle ergometer with continuous respiratory gas and ventilation measurements. Exclusion criteria were a history of pulmonary embolism, significant valvular heart disease, severe obstructive pulmonary disease, exercise-induced angina, and significant ECG changes or the presence of a clinical comorbidity affecting exercise capacity.
Of the total 1042 patients, 155 patients with LVEF >45% were excluded. Of the remaining 887 eligible patients, 43 patients were excluded due to missing MECKI score variables, ultimately resulting in 844 patients enrolled in the study.
On average, the sample of the present study consisted of a younger population, with comparable sex distribution, lower LVEF and peakVO2 (but higher ppVO2), and higher VE/VCO2 slope. Drug treatment was also different, with more patients receiving mineralocorticoid receptor antagonists and fewer receiving digoxin.
Patient follow-up and outcomes
Patients were followed up from 1998 to 2019. Patient follow-up was performed according to the HF programs used at each center. End points were cardiovascular mortality, urgent HTx, or ventricular assist device (VAD) implantation. Patients were considered censored at the time of the endpoint event according to the methods of the original study [4,5].
Metabolic stress test data combined with cardiac and renal index score subgroups.
Patients were divided into three subgroups based on calculated MECKI scores: MECKI score <10%, 10-20%, and ≥20%. Progressive deterioration of clinical parameters, such as by higher New York Heart Association (NYHA) functional class, atrial fibrillation, and VE/VCO2 slope, as well as lower LVEF, peakVO2, and eGFR, was associated with increasing MECKI score values.
Survival rate analysis
In total, there were 263 events: 234 were due to cardiovascular causes (89%: 101 deaths, 58 urgent HTx, and 75 VAD implantations), and 29 were due to noncardiovascular causes (11%), the latter censored at the time of the event.
Study endpoints were recorded in 63 (7.5%), 95 (11.3%), and 122 (14.6%) patients at one, two, and three years, respectively: CV death occurred in 12 (1.4%), 19 (2.3%), and 30 (3.6%); HTx in 24 (2.8%), 37 (4.4%), and 43 (5.1%); and VAD implantation in 27 (3.2%), 39 (4.6%), and 49 (5.8%) at one, two, and three years, respectively. The median event-free survival for the entire sample was 4168 days (11.4 years).
Comparison of survival analysis between the three MECKI score subgroups showed a worse prognosis in patients with a higher MECKI score: median event-free survival was 4396 days (12 years) for a MECKI score <10%, 3457 days (9.5 years) for a MECKI score 10-20%, and 1022 days (2.8 years) for a MECKI score ≥20% (p<0.0001).
Receiver Operating Characteristics Analysis
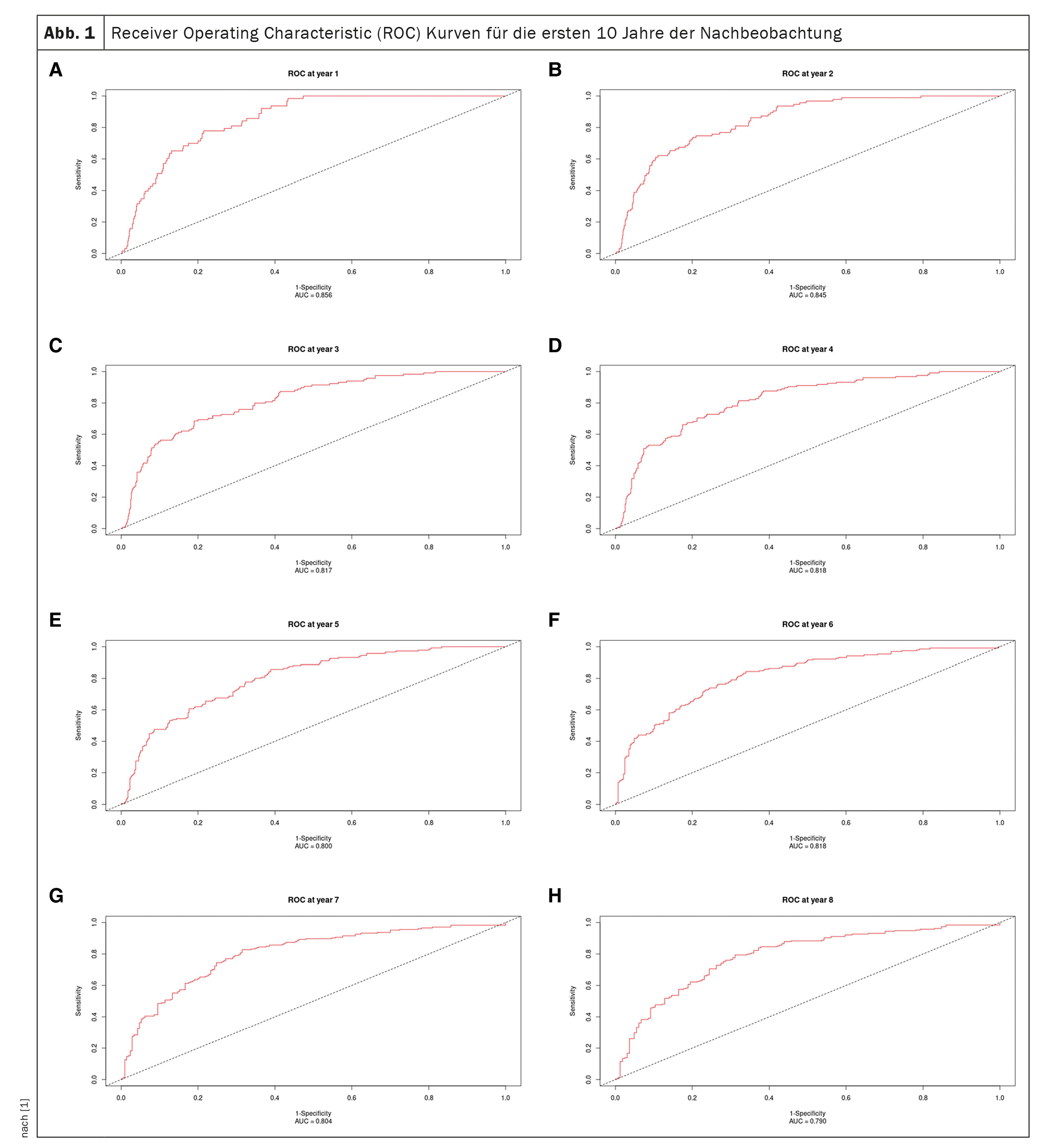
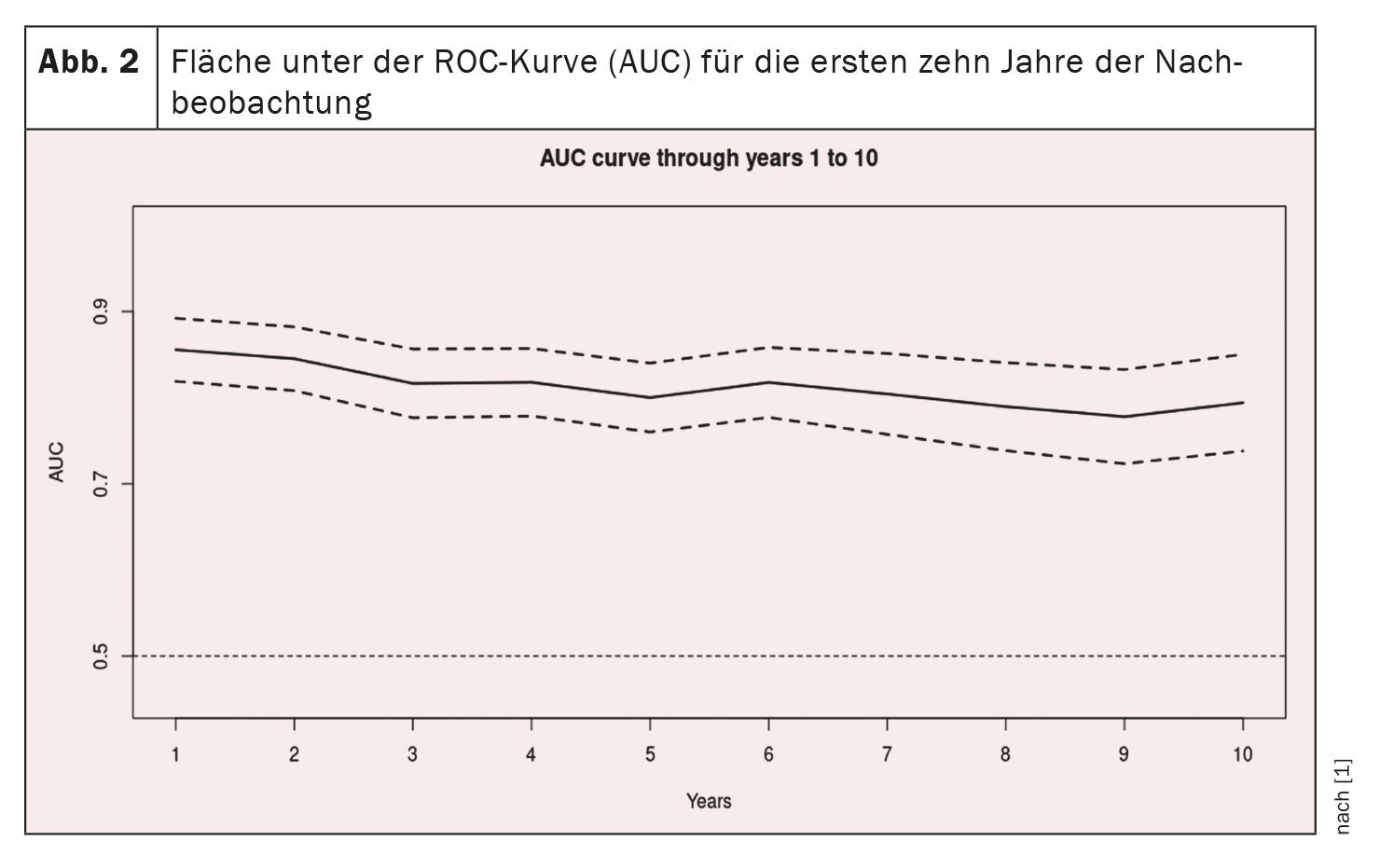
Receiver operating characteristic curves for the first 10 years of follow-up are shown in Figure 1 [1] and the AUC curve inFigure 2 [1]: The AUC remains >0.77 for the 10-year period, but with progressively increasing confidence intervals. AUC values are similar, if not better, than in the original study (0.80 ± 0.02, 0.79 ± 0.01, and 0.76 ± 0.01 at one, two, and three years, respectively) and the validation study (0.81 ± 0.04, 0.76 ± 0.04, and 0.80 ± 0.03 at one, two, and three years, respectively) [12].
Internal and temporal validation show good results for predictive capability
Prognostic stratification in HF patients is essential to guide pharmacological therapy and device implantation. It is also a very useful tool to control the selection of a HTx. In the past, the only scores recommended in this context were the SHFM and the HFSS [13]. The overestimation and underestimation of risk (especially in the highest risk groups) recently demonstrated with the above scores may have a significant impact on treatment decisions, such as the selection of HTx. In patients diagnosed with HFrEF, the MECKI score was confirmed to be informative in terms of prognosis and risk stratification, thus supporting its use as recommended in the HF guidelines.
Take-Home Messages
- Patients diagnosed with heart failure with reduced ejection fraction underwent external validation of the MECKI (Metabolic Exercise Test Data Combined with Cardiac and Kidney Indexes) risk score.
- The prognostic power of the MECKI score was confirmed in a large patient population from Europe and Asia.
- These data support the introduction of the MECKI score as recommended in the European Heart Failure Guidelines 2021.
Literature:
- Adamopoulos S, et al: International validation of the Metabolic Exercise test data combined with Cardiac and Kidney Indexes (MECKI) score in heart failure. Eur J Prev Cardiol 2023; doi: 10.1093/eurjpc/zwad191.
- Bragazzi NL, et al: Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol 2021; 28: 1682-1690.
- Gerber Y, et al: A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015; 175: 996-1004.
- Tsao CW, et al: Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail 2018; 6: 678-685.
- Khush KK, et al: The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult heart transplantation report – 2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019; 38: 1056-1066.
- Salvioni E, et al: The MECKI score initiative: development and state of the art. Eur J Prev Cardiol 2020; 27: 5-11.
- Agostoni P, et al: Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: a multiparametric approach to heart failure prognosis. Int J Cardiol 2013; 167: 2710-2718.
- Agostoni P, et al: Multiparametric prognostic scores in chronic heart failure with reduced ejection fraction: a long-term comparison. Eur J Heart Fail 2018; 20: 700-710.
- Kouwert IJM, et al: Comparison of MAGGIC and MECKI risk scores to predict mortality after cardiac rehabilitation among Dutch heart failure patients. Eur J Prev Cardiol 2020; 27: 2126-2130.
- Freitas P, et al: Comparative analysis of four scores to stratify patients with heart failure and reduced ejection fraction. Am J Cardiol 2017; 120: 443-449.
- Altman DG, et al: Prognosis and prognostic research: validating a prognostic model. BMJ 2009; 338:b605.
- Corra U, et al: The Metabolic Exercise test data combined with Cardiac and Kidney Indexes (MECKI) score and prognosis in heart failure. A validation study. Int J Cardiol 2016; 203: 1067-1072.
- Mehra MR, et al: The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 2016; 35: 1-23.
CARDIOVASC 2023; 22(3): 20-22