In Switzerland, about 32% of all pregnant women have an iron deficiency, which together with the resulting anemia can cause complications for mother and child. Oral and intravenous iron preparations are available for treatment. The aim is to avoid possible transfusions with corresponding risks for mother and child with a timely and adequate therapy of iron deficiency.
As an essential trace element, there is a need to supply the human body with iron from outside at any time in life. However, the need varies according to gender and stage of life, reports Prof. Olav Lapaire, M.D., deputy head of the Department of Medicine. Head of obstetrics in the gynecological clinic at the University Hospital Basel. During pregnancy and the puerperium, iron requirements increase, and thus the recommended iron intake is also significantly higher at 30 and 20 mg/d, respectively, compared with 15 mg/d for women of fertile age [2]. These increased requirements are due to the additional demand for iron by the fetus, the placenta and the expansion of the erythrocyte volume during pregnancy, among others. If left untreated, iron deficiency leads in the course of time to iron deficiency anemia, which still affects 7% of pregnant women in Switzerland. Postpartum, approximately 33% of all women in childbirth have anemia [1].
Diagnostics
The first step in the diagnosis is a detailed medical history and physical examination. This is followed by blood sampling. Here, the Swiss Society of Gynecology and Obstetrics (SGGG) has defined the diagnostic criteria in pregnancy in its Expert Letter No 48 as follows [1]: Iron deficiency exists from a ferritin value of <30 μg/l. Regarding iron deficiency anemia, the company follows the values established by the World Health Organization (WHO): ferritin <30 μg/l and hemoglobin (Hb) of <110 g/l or referring to the recommendations of the Center of Disease Control (CDC), anemia is present in the second trimester from an Hb value of <105 g/l. It is difficult to draw international comparisons or to apply international study results to Swiss practice in this topic, since the ferritin cut-off value in pregnancy is not defined uniformly internationally. The limit value for ferritin in other countries is already <15 μg/l. This diagnostic inhomogeneity makes it difficult to draw conclusions about global prevalence or interpret interventions, Dr. Lapaire said.
In Switzerland, screening for iron deficiency is recommended as part of regular prenatal care. The best time for such testing is when pregnant women regularly present for first-trimester testing between 11 0⁄7 and 13 6⁄7 weeks’ gestation (SSW), the speaker said. At this presentation, blood is usually already drawn for determination of the various serologic parameters routinely monitored in pregnancy, so that ferritin and Hb determination can be easily added. Since ferritin is also an acute phase protein, simultaneous determination of C-reactive protein (CRP) is recommended to exclude falsely high values as far as possible.
Other reference values apply for the diagnosis of postpartum anemia. Here one speaks already starting from a Hb value <120 g/l of anemia. Most often this forms on the basis of pre-existing iron deficiency with the addition of peripartum blood loss. Determination of ferritin level immediately postpartum is not recommended. With regard to its function as an acute phase protein, ferritin can be falsely too high or falsely normal up to six weeks after birth.
In addition to infection-associated anemia, there are other differential diagnostic considerations in anemia workup. As an example, the SGGG expert letter mentions here the substrate deficiency as vitamin B12 or folic acid deficiency and the hemoglobinopathies. The combination of anemia, a normal to elevated ferritin, and altered erythrocyte indices may provide initial clues to any differential diagnoses. If the above-mentioned suspected diagnoses are made, further clarification and, if necessary, an adapted therapy regime are required. However, iron deficiency anemia accounts for the largest proportion of anemias in pregnancy.
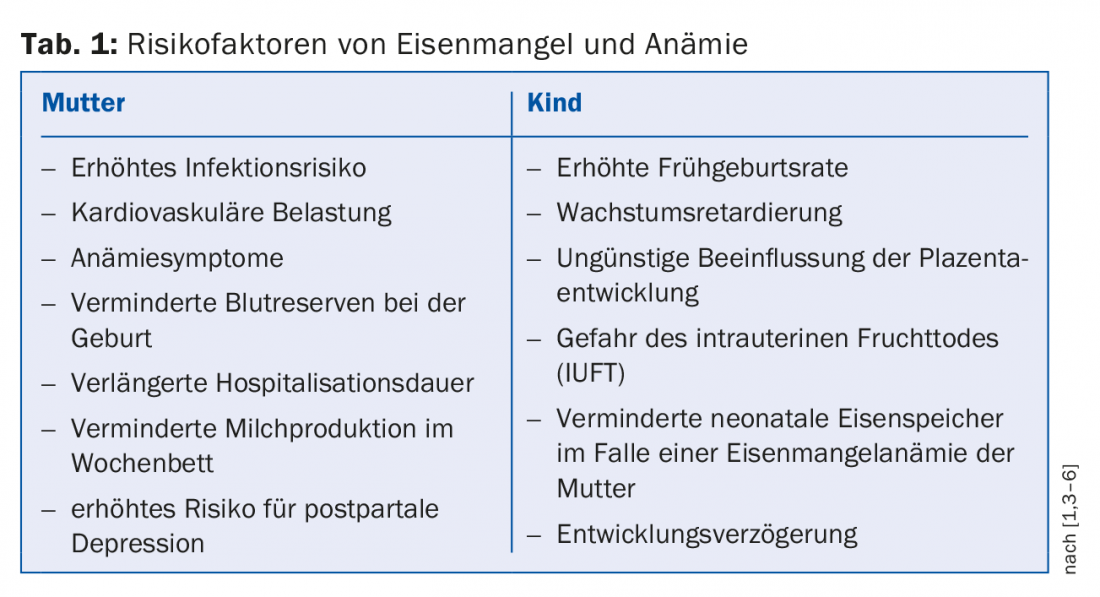
Symptoms and risks
Iron deficiency can manifest itself through various non-specific symptoms. In some cases, these complaints are also described without accompanying anemia. Namely, these include: decreased performance, headaches, fatigue, exhaustion, sensitivity to cold, and an increased risk of developing depression. If anemia is added, the mentioned symptoms can be intensified or additionally dyspnea, dizziness, pallor, brittle nails or rhagades of the corners of the mouth, angina, palpitations or paresthesias of the legs, and others. occur. To alleviate these symptoms, which patients often find distressing, iron supplementation is indicated once the diagnosis is confirmed. In addition to clinical symptoms, it is also important to minimize potential risks of iron deficiency and iron deficiency anemia during pregnancy and postnatally. A distinction is made here between maternal and child factors, which are listed in table 1 .
Therapy
The basic therapeutic options for correcting iron deficiency are oral administration or intravenous (i.v.) administration of an iron preparation. SGGG recommends oral substitution with iron II salts or iron III polymaltoses at a dosage of 160-200 mg/d in case of mild anemia in pregnancy (Hb value 90-110 g/l or 90-105 g/l in the 2nd trimester) or a ferritin value of <30 μg/l without anemia at the beginning of pregnancy. The success of the therapy should be checked after two to four weeks. If the Hb level has not increased by at least 10 g/l after 14 days, if the oral preparation was not tolerated or not taken due to frequent gastrointestinal side effects, advanced anemia with an Hb < 90 g/l or if there is time pressure due to advancing pregnancy or bleeding risks, it is recommended to switch to intravenous therapy. Intravenous therapy is also more commonly advised for certain comorbidities, such as inflammatory bowel disease, which interfere with enteral iron absorption.
In the puerperium, mild anemia is defined as Hb levels between 95-120 g/l, for which peroral iron administration is recommended (80-200 mg iron II salts or iron III polymaltose). If the hemoglobin level falls below 95 g/l, oral administration is not well tolerated, or there are other contraindications to oral substitution, a switch to i.v. administration is also indicated.
Intravenous therapy
Various preparations exist for intravenous iron therapy. Since the launch of the first dextran-containing products, the newer dextran-free infusion solutions have improved tolerability and significantly reduced the risk of anaphylactic reactions [7]. Likewise, bypassing enteral absorption results in a reduction of gastrointestinal side effects [8] and the possibility of extending therapy to patient populations with comorbidities that are contraindications to oral therapy. Dr. Lapaire follows the recommendations of the SGGG and names iron carboxymaltose (Ferinject) for intravenous iron supplementation both in pregnancy and postpartum as the preparation of first choice. This recommendation is based on the results of clinical studies. Breymann et al. were able to show that iron carboxymaltose led to the correction of anemia in a shorter period of time in significantly more pregnant women compared to first-line therapy of orally administered iron, with a concomitant improvement in quality of life [8]. Compared with the also available ferrous saccharate (Venofer), it was shown that a comparably low side effect profile is present [9,10]. Other studies showed that administration of the preparation is also safe for the newborn during breastfeeding [11] and that ferric carboxymaltose cannot cross the placental barrier [12]. Moreover, an additional advantage of ferric carboxymaltose is that it can be given in a dosage of up to 1000 mg, whereas the maximum single dose of ferrous accharate is 200 mg. With Ferinject, multiple costly individual doses can thus be avoided. The preparation is approved from the second trimester and from then on should be adjusted to the weight before the onset of pregnancy. Since there is still a residual risk of anaphylactic reaction even with ferric carboxymaltose, it is recommended by the manufacturer to administer the preparation only in facilities where all devices for resuscitation can be kept ready. Due to possible long-term skin discoloration, special care should be taken to avoid extravasation. The administration of iron substitution should be critically questioned or rejected, for example, in the presence of acute inflammation, indications of liver damage, or risks of iron overload. In summary, as with all drugs administered in pregnancy, a detailed risk-benefit assessment should be made before starting intravenous iron therapy.
If the mother has lost more blood around the time of birth, e.g. in the context of peripartum hemorrhage (PPH), additional administration of erythropoietin (rhEPO) or, in the case of symptomatic anemia, foreign blood administration are available in addition to the administration of iron supplements. However, the goal of timely and adequate therapy of iron deficiency is to avoid possible transfusions and the associated risks.
Source:10th Iron Academy, June 1, 2017, Zurich
Literature:
- Swiss Society of Gynecology and Obstetrics: Diagnostics and Therapy of Iron Deficiency Anemia in Pregnancy and Postpartum. Expert Letter No 48 (replaces No 22) 2017.
- German Nutrition Society: Iron. 2017. www.dge.de/wissenschaft/referenzwerte/eisen/
- Allen LH: Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr 2000; 71(5 Suppl): 1280S-1284S.
- Lelic M, et al: Influence of Maternal Anemia During Pregnancy on Placenta and Newborns. Med Arch 2014; 68(3): 184-187.
- Akhter S, et al: Effect of maternal anemia on fetal outcome. Mymensingh Med J 2010; 19(3): 391-398.
- Radlowski EC, Johnson RW: Perinatal iron deficiency and neurocognitive development. Front Hum Neurosci 2013; 7: 585.
- Cançado RD, Muñoz M: Intravenous iron therapy: how far have we come? Rev Bras Hematol Hemoter 2011; 33(6): 461-469.
- Breymann C, et al: Ferric carboxymaltose vs. oral iron in the treatment of pregnant women with iron deficiency anemia: an international, open-label, randomized controlled trial (FER-ASAP). J Perinat Med 2016; pii: /j/jpme.ahead-of-print/jpm-2016-0050/jpm-2016-0050.xml.
- Christoph P, et al: Intravenous iron treatment in pregnancy: comparison of high-dose ferric carboxymaltose vs. iron sucrose. J Perinat Med 2012; 40(5): 469-474.
- Pfenniger A, et al: Safety and efficacy of high-dose intravenous iron carboxymaltose vs. iron sucrose for treatment of postpartum anemia. J Perinat Med 2012; 40(4): 397-402.
- Bailie GR: Efficacy and safety of ferric carboxymaltose in correcting iron-deficiency anemia: a review of randomized controlled trials across different indications. Drug Research 2010; 60(6a): 386-398.
- Malek A: In vitro studies of ferric carboxymaltose on placental permeability using the dual perfusion model of human placenta. Drug Research 2010; 60(6a): 354-361.
HAUSARZT PRAXIS 2017; 12(7): 36-38











