What benefits can patients with relapsing-remitting multiple sclerosis (RMS) expect from first-line treatment with ofatumumab (KESIMPTA®)? The answer to this question was provided by a subgroup analysis of data from treatment-naive patients in the two ASCLEPIOS trials. Here, patients on ofatumumab were shown to have lower disease activity and slower progression compared with teriflunomide. Tolerability was comparable to the overall study population and adherence was correspondingly high.
A growing body of data suggests that early use of high-efficacy therapy (HET) has a more positive impact on disease progression than starting with a moderately effective option and escalating therapy as disease worsens. For example, an analysis of 592 British patients found that early use of HRT compared with escalation therapy over a five-year period resulted in better outcomes in terms of annual relapse rate, Expanded Disability Status Scale (EDSS) score, and sustained disability accumulation.1 Monoclonal antibodies were considered to be HET, and all other disease-modifying therapies were assigned moderate efficacy. Furthermore, in a retrospective analysis, 213 patients with early HRT (started within 2 years of disease onset) were compared with a matched group of 253 patients in whom HRT was not started until 4 to 6 years after disease onset.2 This showed that patients in whom HRT was started early had a significantly lower mean EDSS score at 6 to 10 years than patients in whom HRT was started later (2.3 vs. 3.5 at 10 years; p < 0,0001). However, such early options should also have a good safety profile and not burden patients and their adherence with frequent treatment and follow-up appointments and any necessary pretreatment.
ASCLEPIOS I and II: subgroup analysis of therapy-naïve patients.
The fully human anti-CD20 antibody ofatumumab provides a new, highly effective option for the first-line treatment of RMS.3,4 The efficacy and safety of ofatumumab in the treatment of patients with RMS were evaluated in the two identically designed, multicenter, double-blind, active-controlled, double-dummy, parallel-group studies, ASCLEPIOS I (n = 927) and ASCLEPIOS II (n = 955).5 Teriflunomide was chosen as the comparator. Included patients in the ofatumumab arm received a starting dose of 20 mg ofatumumab subcutaneously (s.c.) on days 0, 7, and 14, followed by a monthly maintenance dose (also 20 mg) and placebo by oral route. Maintenance therapy could be self-applied by patients at home. The comparison group was treated with 14 mg teriflunomide per day and placebo s.c.. The duration of the study was flexible and was a maximum of 30 months.
In a subgroup analysis, the benefit-risk profile of treatment with ofatumumab was investigated in beitherapienaive patients with early-stage disease (≤ 3 years since diagnosis).6 This was approximately one-third of the total ASCLEPIOS population (314 patients on ofatumumab, 301 patients on teriflunomide). The median age of these patients was 36 years and the median time since diagnosis was 0.35 years.
Therapy-naïve patients: Results consistent with ASCLEPIOS overall population.
Analysis of data from treatment-naïve patients showed that treatment with ofatumumab resulted in a significant reduction in annualized relapse rate (ARR) of 50.3% compared to teriflu- nomide (p < 0,001).6 In addition, 6-month confirmed disability worsening (CDW) was reduced by 46% with ofatumumab compared with teriflunomide (p=0.044). This result, as well as the 3-month CDW result (38% risk reduction; p=0.065), was consistent with that of the overall population. Analysis of various imaging parameters confirmed the superiority of ofatumumab (Fig. 1). Thus, the number of gadolinium (Gd)-positive T1 lesions per scan was significantly reduced by 95.4% by ofatumumab compared with teriflunomide (p <0.001). The number of new/enlarging T2 lesions also decreased significantly by 82.0% per year (p < 0.001). In the second year of treatment, ofatumumab even showed a 97.1% reduction in T2 lesions compared to teriflunomide (p < 0.001).
 Fig. 1. ofatumumab achieved significantly better results in therapy-naïve MS patients compared with teriflunomide on several imaging parameters.6
Fig. 1. ofatumumab achieved significantly better results in therapy-naïve MS patients compared with teriflunomide on several imaging parameters.6
One of the goals of successful MS treatment is NEDA (No Evidence of Disease Activity).7 Here, NEDA-3 is defined by the absence of relapses (1), disability progression (2), and radiographic activity (3). As it turned out, the chance to reach NEDA-3 by treatment with ofatumumab was more than 3-fold higher for the therapy-naive patients in the two ASCLEPIOS studies in the first year of treatment and even more than 14-fold higher in the second year of treatment than under teriflunomide (Fig. 2).6
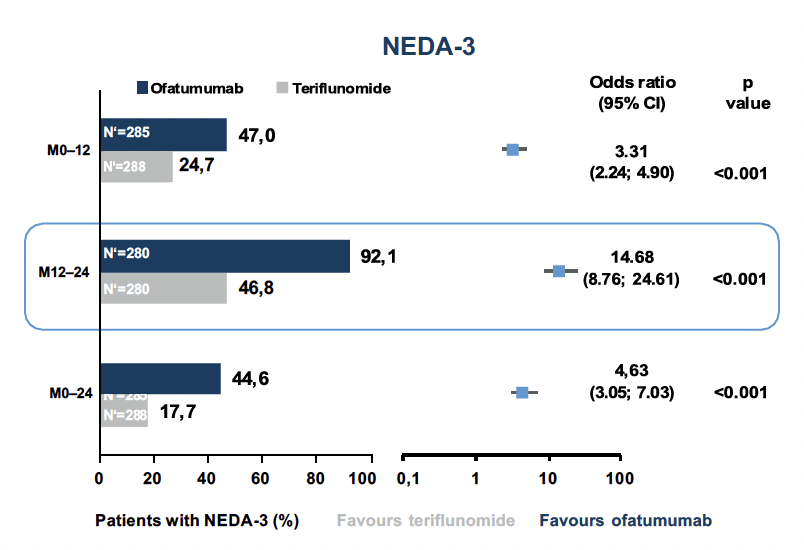 Fig. 2. there was a significantly higher chance of achieving NEDA-3 with ofatumumab than with teriflunomide treatment.6
Fig. 2. there was a significantly higher chance of achieving NEDA-3 with ofatumumab than with teriflunomide treatment.6
Favorable side effect profile
The analysis of side effects made it clear that the good efficacy of ofatumumab is not at the expense of tolerability. In the overall population of the two ASCLEPIOS studies, adverse events occurred in 83.6% of patients on ofatumumab and 84.2% of patients on teriflunomide.5 Serious events were recorded in 9.1% and 7.9% of patients, respectively. Injection-associated reactions were most common. They occurred in 20.2% of patients in the ofatumumab group and 15.0% of patients in the teriflunomide group. Systemic reactions to injection were mild or moderate in more than 99% of cases. They occurred mainly after the first injection and decreased significantly with subsequent applications. Infections were recorded in 51.6% of ofa- tumumab patients and 52.7% of teriflunomide-treated patients. Na- sopharyngitis (18% ofatumumab, 16.7% teriflunomide), upper respiratory tract infections (10.3% vs. 12.8%), and urinary tract infections (10.3% vs. 8.3%) were most common.
Analysis of the treatment-naive patient group showed a balanced incidence of adverse events between the two treatment groups.6 The rate of serious adverse events was lower in the treatment-naïve group than in the overall population, but remained comparable between ofatumumab and teriflunomide groups. No cases of opportunistic infections such as PML (progressive multifocal leukoencephalopathy) or hepatitis B reactivation have been recorded. Adherence with ofatumumab in patients receiving therapy was high (98.8%) and almost identical to adherence in the ASCLEPIOS overall population (98.3%).
Conclusion
More and more data suggest that early use of highly effective therapy has a more positive impact on disease progression than escalating therapy when the disease worsens. However, highly effective therapies are often associated with a limited safety profile. As the two ASCLEPIOS studies showed, ofatumumab proved to be highly effective not only in the overall population, but especially in previously untreated patients at an early stage of the disease. In addition, the side effect profile in the therapy-naïve patients was comparably favorable as in the already pretreated patients. After initiation of therapy under the supervision of the treating physician, patients can easily self-administer KESIMPTA® (ofatumumab), once a month with a subcutaneous autoinjector pen. In addition, no premedication, no hospital stay, and no subsequent observation phase are necessary during the application. Adherence of treatment-naïve patients as well as the overall population in the ASCLEPIOS studies was over 95%.
This article was realized by Novartis Pharma Schweiz AG, Suurstoffi 14, 6343 Rotkreuz, Switzerland
NO55104/04.2021