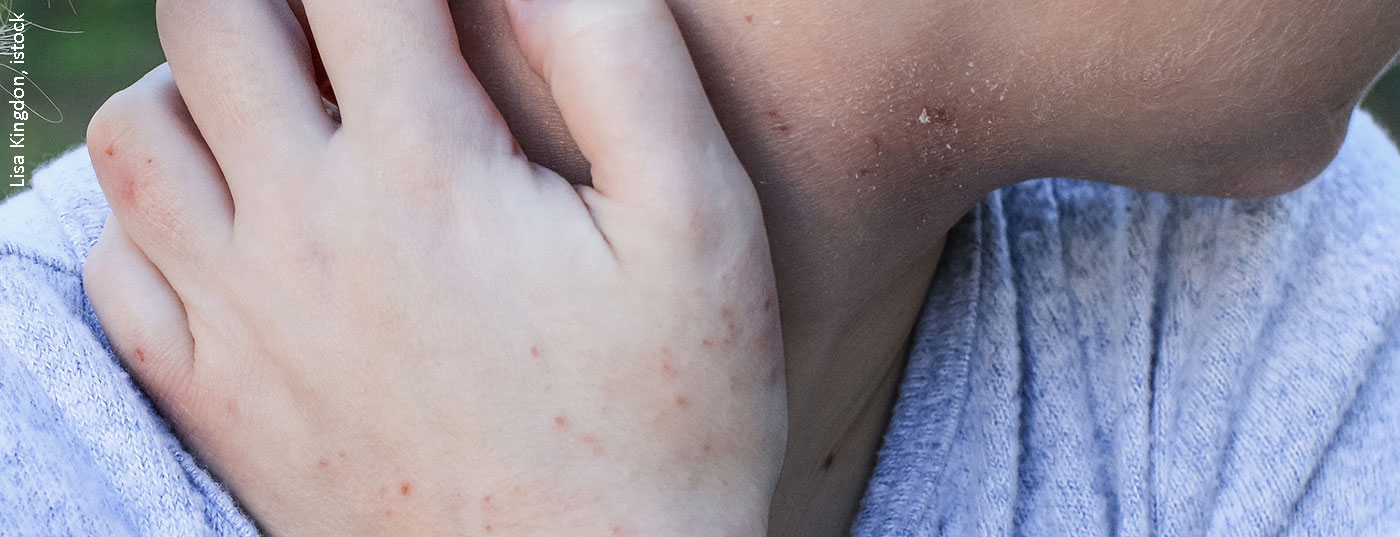
In adult patients with moderate to severe atopic dermatitis, long-term therapy with the JAK inhibitor baricitinib maintained improvements achieved after 16 weeks for 68 weeks, new data show. A pooled analysis of multiple randomized-controlled trials, also recently published, also demonstrated a favorable safety profile.
In contrast to cortisone or ciclosporin, biologics and JAK inhibitors specifically influence individual activation cascades of the immune system. This explains the high efficacy and the comparatively favorable side effect profile, which is significant in view of the chronic-recurrent course of atopic dermatitis. The oral selective Janus kinase (JAK) inhibitor baricitinib (Olumiant®) was approved in Switzerland in February 2021 for the treatment of adult patients with moderate to severe atopic dermatitis. To date, this is the first oral JAK inhibitor for the treatment of atopic dermatitis. Within the intracellular signaling pathway, JAKs phosphorylate and activate signal transducers and activators of transcription (STATs), which in turn activate gene expression within the cell. Baricitinib modulates these signaling pathways by partially inhibiting the enzymatic activity of JAK1 and JAK2, thereby reducing phosphorylation and activation of STATs.
JAK inhibitors block multiple cytokines simultaneously
The pathophysiology of atopic dermatitis is complex, with a type 2 immune response mediated by activated Th2 lymphocytes playing a major role. Possible trigger factors include allergens, microbial components, or other environmental antigens. In cytokine signaling, the JAK-STAT pathway plays a critical role. When a cytokine binds to the extracellular domain of its receptor, JAK molecules activate intracellular transcription factors that control gene readout in the nucleus [1]. The mechanism of action of JAK inhibitors in atopic dermatitis is based on the inhibition of numerous cytokines that play a central role in pathogenesis. Baricitinib selectively inhibits the Janus kinases JAK1 and JAK2. Through JAK1, the signals of key adaptive and innate cytokines IL-4, IL-13, IL-22, IL-31, TSLP, IFN-γ, and IL-6 are transmitted, and JAK2 is necessary for the function of the IL-5 receptor, which is required in the recruitment of eosinophilic granulocytes [2]. To date, JAK inhibitors have been particularly convincing in their very rapid onset of itch relief, with significant improvement as early as week 1 after initiation of therapy [3].
Extension study shows sustained efficacy of barictinib over 68 weeks
The efficacy and safety of baricitinib over a 16-week period in patients over 18 years of age with moderate to severe atopic dermatitis* was demonstrated in the BREEZE-AD1 and -AD2 studies, among others [3]. Study participants had previously demonstrated an inadequate response or intolerance to topical medications. Olumiant® has been used as monotherapy or in combination with topical corticosteroids (TCS). To investigate the long-term effects in adults, an extension study was conducted based on BREEZE-AD1 and -AD2. Those who had a complete or partial response to therapy in these two preliminary studies were enrolled in the multicenter, double-blind BREEZE-AD3 trial for an additional 52 weeks (Table 1) [3,4]. Patients with a vIGA-AD score of 0 or 1 who did not require emergency therapy were considered complete responders. The corresponding criterion for partial responders was vIGA-AD 2, and also no requirement for emergency therapy. Similar to previous studies, study participants received baricitinib 2 mg (n=54) or 4 mg (n=70).
* IGA ≥3, EASI ≥16, and BSA ≥10%.
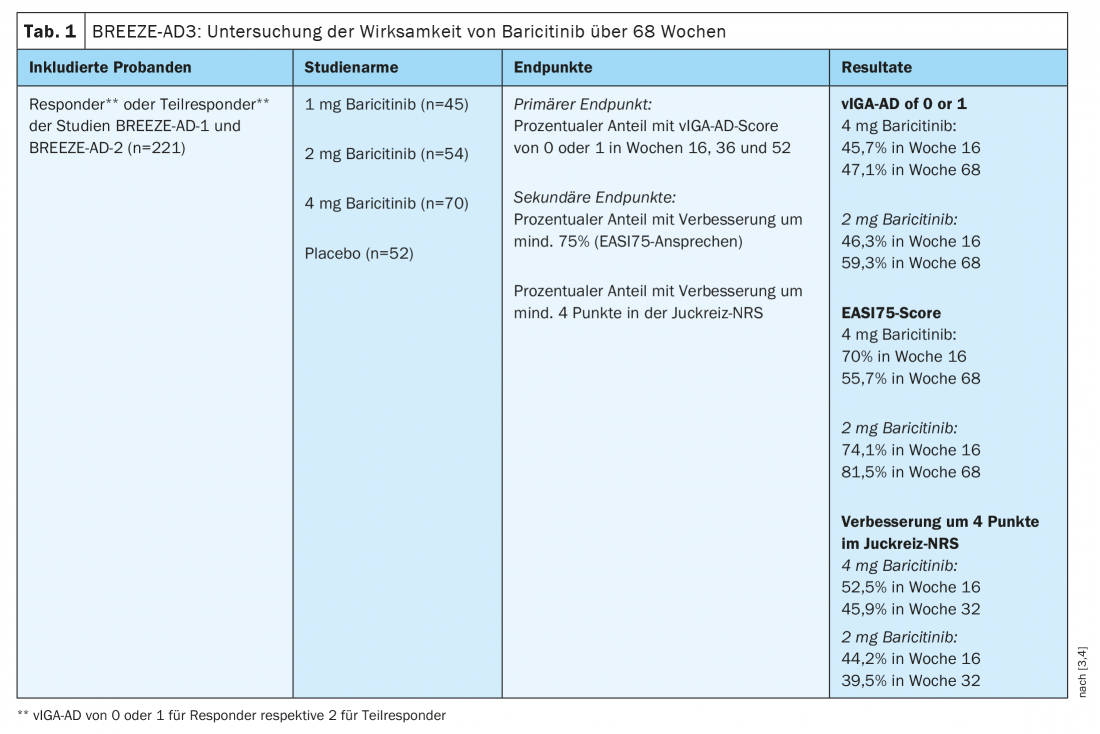
In the 4-mg group, vIGA-AD proved stable under continuous therapy throughout the study period. At week 16, 45.7% of patients had a score of 0 or 1, and at week 68, this score was 47.1%. An EASI75 response was achieved by 70.0% of participants overall at week 16, whereas this proportion was 55.7% at week 68. A reduction in itch of at least four points on the numerical ranking scale (NRS) was documented in 52.5% of study participants at week 16 and in 45.9% at week 32. In the 2-mg group, 46.3% achieved vIGA-AD of 0 or 1 at week 16 and 59.3% at week 68. Regarding proportion of patients with EASI75, there was an increase from 74.1% to 81.5%. A reduction in itching by at least. 4 points in the NRS was registered at 44.2% at week 16 and 39.5% at week 32.
Pooled data attest to favorable safety profile
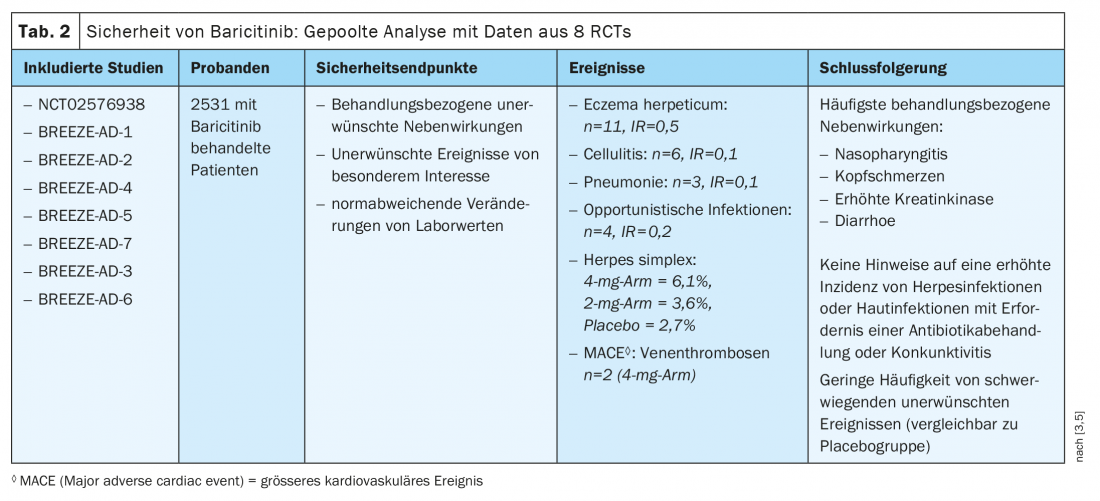
The recently published results of a pooled safety analysis of baricitinib in adult patients with atopic dermatitis is based on 8 randomized clinical trials (Table 2) [3,5]. Included were data from six double-blind RCTs (NCT02576938, BREEZE-AD-1, BREEZE-AD-2, BREEZE-AD-4, BREEZE-AD-5, BREEZE-AD-7) and two extension studies (BREEZE-AD-3, BREEZE-AD-6). The results show a frequency of serious adverse events comparable to placebo. The most common treatment-related adverse events reported were nasopharyngitis, headache, and elevation of creatine phosphokinases and diarrhea. During the first 16 weeks, an increase in herpes simplex infections was recorded compared with placebo, although there was no evidence that continuous baricitinib therapy was associated with an increased incidence of herpes infections. In addition, there was no increase in skin infections requiring antibiotic treatment, no increase in serious cardiovascular events, and no increase in conjunctival symptoms.
In summary, the data from the BREEZE AD-3 study and the pooled safety analysis support that baricitinib is a valid systemic therapy alternative in adults with atopic dermatitis, particularly for those patients who prefer an oral dosage form. The efficacy and safety of baricitinib in children and adolescents is currently being evaluated in the BREEZE-AD-PEDS study.
Literature:
- Solimani F, Hilke FJ, Ghoreschi K: Pharmacology of Janus kinase inhibitors. Dermatologist 2019; 70 (12): 934-941.
- Lauffer F, Biedermann T: Dtsch Arztebl 2021; 118(24): [24]; DOI: 10.3238/PersDerma.2021.06.18.04
- Melo A, et al: Baricitinib for the treatment of atopic dermatitis. Journal of Dermatological Treatment 2021, https://doi.org/10.1080/09546634.2021.1967268
- Silverberg JI, et al: Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or par- tial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157(6): 691-699.
- Bieber T, et al: Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J Eur Acad Dermatol Venereol. 2021;35(2): 476-485.
DERMATOLOGIE PRAXIS 2021; 31(5): 38-40