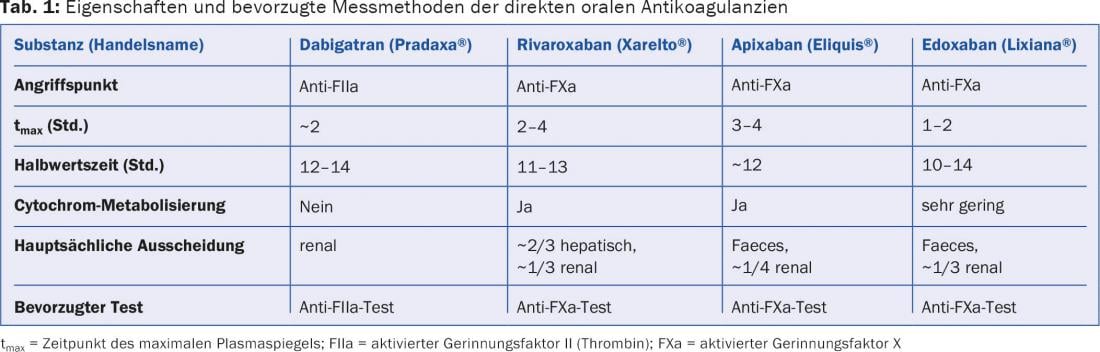
DOAK affect routine coagulation tests to an extent that varies depending on the substance, the time of measurement, and the test system used. However, a statement about the strength of anticoagulation, which is usual from conventional anticoagulants, cannot be derived from this; this requires a test calibrated for the respective DOAK. In our experience, methods of choice are thrombin time modifications for dabigatran and chromogenic anti-factor Xa assays for the factor Xa antagonists (rivaroxaban, apixaban, edoxaban). A target range of anticoagulant effect does not exist. The possibility of falsification of other coagulation parameters should be considered.
The anticoagulants dabigatran etexilate (Pradaxa®), rivaroxaban (Xarelto®), apixaban (Eliquis®), and edoxaban (Lixiana®), which are still frequently referred to as “new” anticoagulants, are direct oral anticoagulants (DOAs) that do not rely on antithrombin mediation for their action. In contrast, unfractionated and low-molecular-weight heparins, the heparinoid danaparoid (Orgaran®) and the pentasaccharide fondaparinux (Arixtra®) are only effective in the presence of antithrombin, i.e. indirectly. Vitamin K antagonists (VKA) such as phenprocoumon (Marcoumar®) or acenocoumarol (Sintrom®), in turn, interfere with the vitamin K-dependent synthesis of certain coagulation factors.
DOAK are increasingly used for prophylaxis and therapy of thromboembolism. Questions concerning the handling of these substances in complex situations are accordingly gaining in importance. In the following, the laboratory methods commonly used to measure their effect and indications for their use will be presented.
When is measurement of anticoagulant effect of interest?
The currently available DOAKs belong to two substance classes (Table 1):
- Oral direct thrombin (factor IIa) antagonists with dabigatran etexilate (Pradaxa®) as the only representative to date.
- Oral direct factor Xa antagonists. From this class, rivaroxaban (Xarelto®), apixaban (Eliquis®) and edoxaban (Lixiana®) are available in Switzerland.
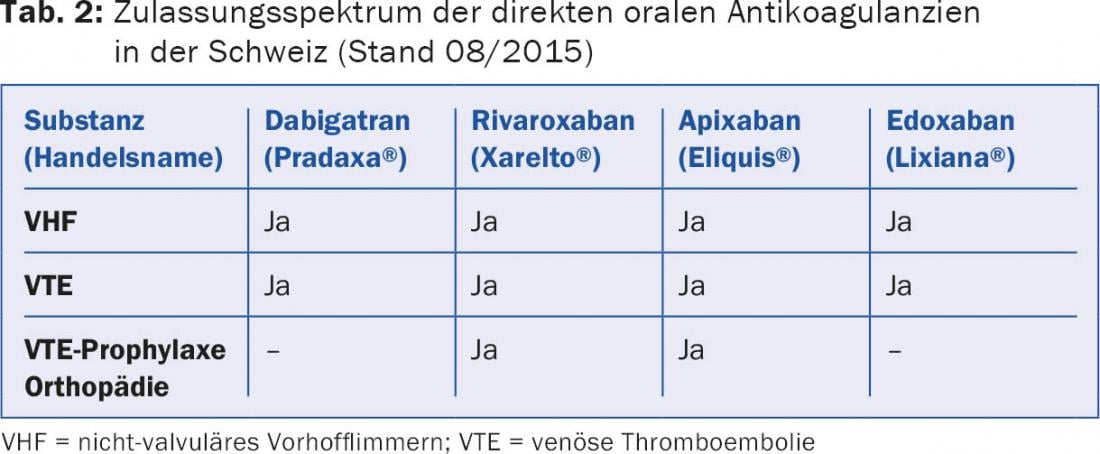
The spectrum of approved indications currently includes thromboembolism prophylaxis after total hip and total knee arthroplasty surgery, embolic prophylaxis in nonvalvular atrial fibrillation, and therapy and secondary prophylaxis of venous thromboembolism, but not thromboembolism prophylaxis in medical or general surgical patients or in patients with mechanical prosthetic heart valves (Table 2).
Because the pharmacokinetics and pharmacodynamics of DOAs are highly predictable and the potential for drug-drug interactions is comparatively low, they are administered according to a fixed dosing schedule. Monitoring of their anticoagulant effect, as is essential for VKA, is not usually required but may be of interest in certain circumstances [1]:
- Short-term procedures and interventions if the last medication was taken less than 24 hours ago (longer if necessary in the case of impaired renal function)
- Perioperative management
- Antagonization of the anticoagulant effect
- Bleeding
- Body weight extremes
- Deterioration of kidney function
- Suspected overdose
- Suspected drug interactions
- Occurrence of thrombosis or bleeding under adequate therapy.
In our opinion, measurement is not suitable for checking compliance – if only in view of the short half-life of the new substances (Tab. 1).
Interpretation of the measurement
There are several points to consider when interpreting the measured values:
- A target range, as is common for anticoagulation with VKA or heparins, does not exist for DOAK.
- Routine coagulation tests such as Quick/INR, activated partial thromboplastin time (aPTT), and thrombin time are affected by DOAK to varying degrees; however, this does not allow quantification of their anticoagulation effect. Other coagulation parameters may also be distorted. The nature and strength of these influences are variable and are largely determined by the drug’s point of action, the interval between its last ingestion and blood collection, and the reagents and test systems used. It is recommended to evaluate the latter point under the conditions of your own laboratory.
- Time of measurement: For the measurement of the maximum plasma concentration of a DOAK, the blood sample should be taken approximately three hours after its intake (Tab. 1), for that of the trough level after 12-24 hours. By its nature, the effect on coagulation tests is greatest at the time of maximum plasma concentration.
Dabigatran etexilate
As a direct thrombin antagonist, dabigatran affects thrombin time most strongly of the routine tests, followed by aPTT, and to a lesser extent Quick/INR. At dabigatran plasma concentrations of 100 ng/ml, prolongation of thrombin time and aPTT can be expected, while Quick is typically still within the normal range. These influences vary depending on the reagent used [2]. An example of a comparatively sensitive aPTT reagent is Pathromtin SL®.
The most common assays for quantifying dabigatran effects are based on modifications of thrombin time calibrated with dabigatran concentration standards. They are sensitive in the concentration range between 50-1000 ng/ml. An example of a commercially available test is the Hemoclot® assay.
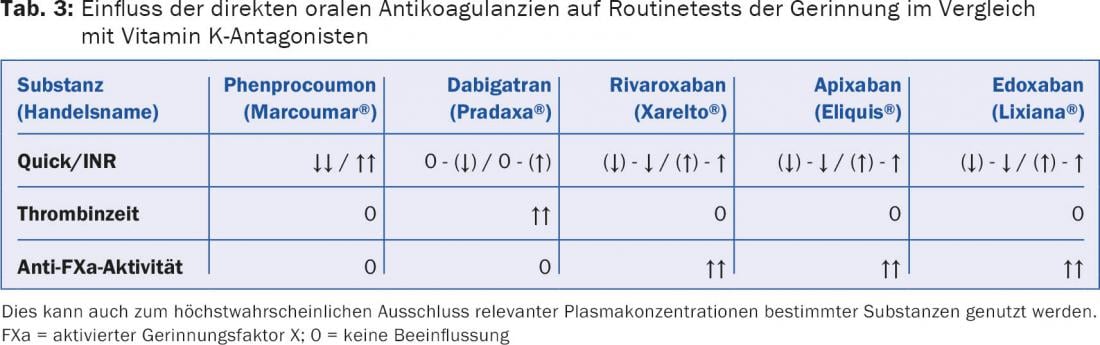
Routine thrombin time is useful as a sensitive test to exclude significant dabigatran concentration. It can be used for guidance when a quantitative test is not available (Table 3).
Since the most common type of fibrinogen measurement according to Clauss methodologically represents a modification of the thrombin time, this is also influenced by dabigatran.
This may result in a prolongation of the clotting time and consequently in an underestimation of the fibrinogen concentration.
Methods for antithrombin measurement that use thrombin inhibition as an end point may also be influenced by dabigatran (overestimation of antithrombin levels). In contrast, methods based on factor Xa inhibition are not biased.
Rivaroxaban, apixaban, edoxaban
A maximum plasma level is measured about two to four hours after intake (tab. 1). In addition to the Quick test and INR, the direct factor Xa antagonists also influence the aPTT to a lesser extent, but primarily, of course, all anti-factor Xa methods, such as those used to monitor unfractionated or low-molecular-weight heparins. In contrast to dabigatran, factor Xa antagonists do not affect thrombin time [3] (Table 3).
The extent to which Quick test and INR are affected can vary significantly depending on the test system and thromboplastin reagent. An example of a comparatively sensitive reagent is Neoplastin®, and a less sensitive one is Innovin®.
The most common methods for quantifying anticoagulant activity that correlate best with plasma concentration are chromogenic anti-factor Xa assays, as used for heparins. These are calibrated using concentration standards that are also commercially available (for example, from Hyphen Biomed, Technoclone or Stago).
If a quantitative, specifically calibrated anti-factor Xa method is not available, a routine anti-factor Xa method (heparin) is suitable as an orienting and exclusion test (Table 3).
As mentioned, the thrombin time is not influenced by the direct factor Xa antagonists; this is therefore also true for the fibrinogen measurement according to Clauss. Antithrombin assays that use thrombin inhibition as an end point are also unaffected-in contrast, those with an anti-factor Xa end point may result in an overestimation of antithrombin levels.
Other factor X-dependent tests may also be confounded [4]:
- Dilute Russel viper venom time (dRVVT) as a test for a lupus anticoagulant may be false-pathologic.
- Tests for resistance to activated protein C (APC resistance in factor V Leiden mutation) may be false-normal.
- Tests measuring protein C and protein S function may be false-normal.
If necessary, the measurement should be repeated after cessation of anticoagulant therapy or at the time of a minimal plasma level of the factor Xa antagonist 12-24 hours after the last dose. Since the laboratory is often not informed when analyses are requested whether an anticoagulant is being taken and, if so, which one and at what interval from sample collection, our laboratory always measures anti-factor Xa activity for critical parameters to ensure the validity of the result.
Literature:
- Baglin T, et al: Measuring oral direct inhibitors of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2013; 11: 756-760.
- Harenberg J, et al: Determination of dabigatran in human plasma samples. Semin Thromb Hemost 2012; 38: 16-22.
- Mani H, et al: Rivaroxaban differentially influences ex vivo global coagulation assays based on the administration time. Thromb Haemost 2011; 106: 156-164.
- Hillarp A, et al: Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost 2011; 9: 133-139.
HAUSARZT PRAXIS 2015; 10(9): 27-29