For patients with axial spondyloarthritis (axSpA), in whom therapy with non-steroidal anti-inflammatory drugs was not effective, several active agents from the substance classes of biologics and JAK inhibitors are available today. In addition to efficacy, an important selection criterion is the safety profile of the DMARD. Furthermore, the patient’s preferences should be taken into account. The ASAS-EULAR recommendations also provide guidance on how to proceed with a change in therapy.
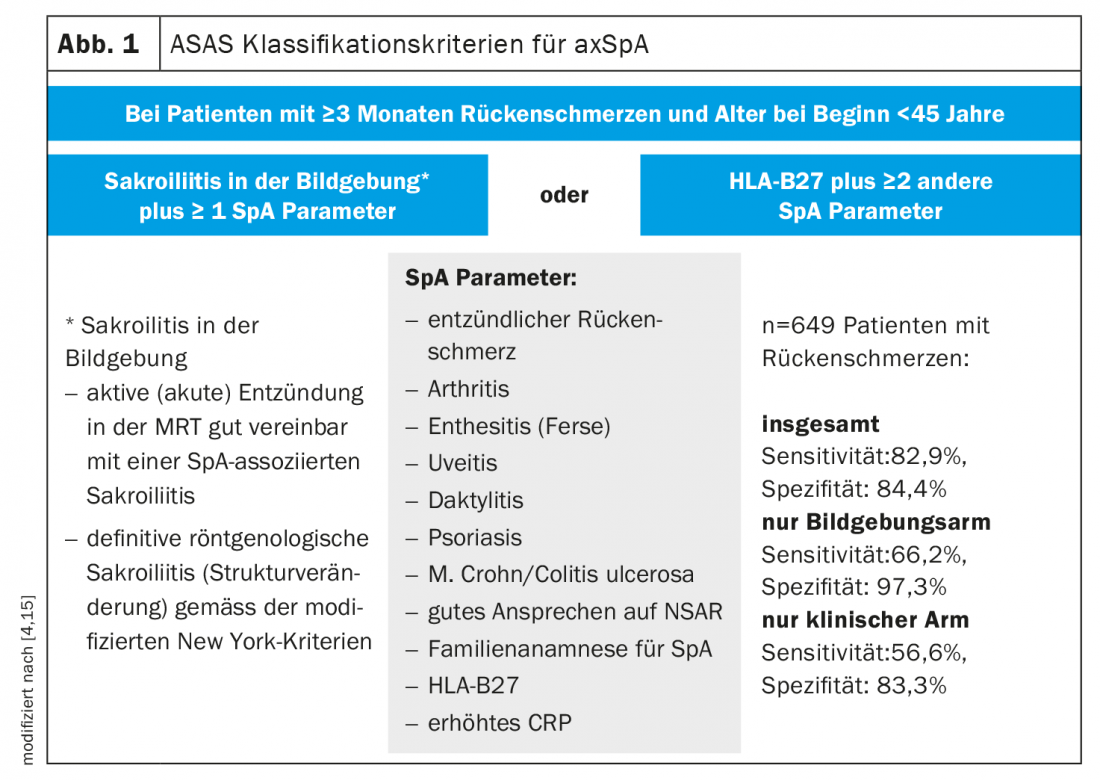
At this year’s EULAR Annual Meeting, renowned experts discussed the latest findings on the diagnosis and treatment of axial spondyloarthritis (axSpA). AxSpA is an inflammatory spinal disease of the rheumatic type that may be associated with various musculoskeletal and extraskeletal manifestations [1]. The cardinal symptom of axSpA is inflammatory back pain. Diagnostic workup includes radiographic and MRI examination of the lumbar spine (LS) and sacroiliac joints, and laboratory screening that includes inflammatory markers (ESR, CRP) and testing for HLA-B27 [2]. The history should include exploration of psychosocial stress/work-related stress in addition to extra-articular manifestations. The ASAS (“Assessment of SpondyloArthritis International Society”) classification criteria were established to confirm the diagnosis (Fig. 1) [3]. These require mandatory evidence of HLA-B27; another criterion is sacroiliitis detectable by imaging. Those patients without structural changes are referred to as nonradiographic axial SpA, whereas patients with structural changes in the sacroiliac joints are classified as having ankylosing spondyloarthritis (ankylosing spondylitis). To assess disease activity, in addition to results of clinical examination, imaging, and laboratory diagnosis, the BASDAI (Bath Ankylosing Spondylitis Disease Activity Index) and the more recent ASDAS (Ankylosing Spondylitis Disease Activity Score), which is based on some BASDAI questions and includes CRP, are available [4–7]. Disease activity should be assessed at regular intervals, usually every 3 to 6 months, using clinical composite scores [3,5].
Multimodal treatment concept
A combination of non-pharmacological measures (e.g., exercise therapy) and drug therapy is recommended for the treatment of patients with axSpA [1]. In addition to pain reduction, the goal is to preserve physical functioning and prevent structural lesions [5,8,9]. The goals of drug treatment are primarily to reduce disease activity and achieve clinical remission. This includes pain reduction, reduction of inflammatory processes, and inhibition of radiographic progression. Nonsteroidal anti-inflammatory drugs (NSAIDs) are still considered first-line therapy for axial SpA [5]. Due to the safety profile of NSAID preparations, the dosage and duration of therapy should be continually reviewed. Disease modifying anti-rheumatic drugs (DMARDs) can be used in patients who do not achieve sufficient reduction in inflammatory disease activity with standard NSAID therapy.
Biologics and JAK inhibitors – what is the current state of knowledge?
Prof. Désirée van der Heijde, MD, Leiden University Medical Center (NL), provided an update on bDMARD and tsDMARD as treatment options for axSpA [10]. One of the innovations in the ASAS-EULAR recommendations for the management of axSpA, updated in 2022, is that the ASDAS criteria (Fig. 1) are primarily used to assess disease progression, and the BASDAI criteria have become less important. When persistent high disease activity is present despite NSAID therapy, with an ASDAS score ≥2.1, it is suggested that therapy with a TNFα inhibitor (TNFα-i), IL-17 inhibitor (IL-17-i), or Janus kinase inhibitor (JAK-i) be initiated. Although efficacy data for biologics and JAK-i are similar, it is generally advised to first attempt therapy with TNFα-i or IL-17-i. “Safety considerations are predominant in the preference of TNFα inhibitors and IL-17 inhibitors over JAK inhibitors,” the speaker explained.
Regarding the IL-17-i secukinumab, data from extension phase randomized-controlled trials show a low rate of serious infections, malignancies, and cardiovascular events. Regarding ixekizumab, also an IL-17-i, data are scarce. This is also true for JAK-i, where only data from short-term RCTs are available.
In patients with rheumatoid arthritis (RA) and certain risk factors, data from the Oral Surveillance study indicate that JAK-i are associated with an increased risk of cardiovascular events (MACE) and malignancies. The speaker emphasized that the RA patient population is not the same as the axSpA population [10,14]. Nevertheless, she also urges caution in patients with axial SpA and certain risk factors, given the paucity of data regarding JAK-i. In any case, the benefits and risks must be carefully weighed up in each individual case.
Table 1 summarizes the efficacy of bDMARDs and tsDMARDs on various manifestations of axSpA. Observational studies indicate that adalimumab, infliximab, golimumab, and certolizumab pegol have higher efficacy with respect to uveitis than secukinumab and etanercept [11]. With regard to the JAK inhibitors tofacitinib and upadacitinib on this no data are available.

Treatment attempt with a DMARD failed – what next?
On the question of what to do if treatment with a bDMARD was not effective, Prof. van der Heijde advises: “Then the first thing to do is to reconsider the diagnosis. This is especially true when patients with back pain have not responded to therapy with either TNFα-i or IL-17-i. If there is high disease activity, one should ask if there are other comorbidities or patient characteristics that might influence this. In such cases, an MRI is recommended to determine if there is evidence of inflammation in the spine. What is the specific recommendation of the updated ASAS-EULAR recommendations on this? This states that after an initial failed treatment attempt with bDMARD or tsDMARD, switch to another bDMARD (TNFα-i or IL-17-i) or a JAK-i. Overall, the data on switching therapy after a failed treatment attempt with one or more bDMARDs or tsDMRADs is rather limited at this point, she said. With regard to secukinumab, there are data showing that good response rates are achieved in patients who have had a failed treatment attempt with TNFα-i, although response rates tended to be better in biologics-naive patients [12]. The same pattern was seen in corresponding studies on ixekizumab [13].
The speaker emphasized that it is always important to take into account the overriding general principles of therapy when treating patients with axSpA. Decision making for the best possible treatment option should be done together with the patient (“shared decision making”). Individual and social criteria must also be taken into account. If necessary, the form of application (sc, iv, oral) can be a criterion for selecting the most appropriate treatment in each case, in addition to efficacy, safety and cost factor.
Congress: EULAR Annual Meeting
Literature:
- Kiltz U, et al: Long version of the S3 guideline Axial spondyloarthritis including ankylosing spondylitis and early forms, update, Z Rheumatol 2019(78): 3-64.
- Rudwaleit M: Spondyloarthritides. Z Rheumatol 2017; 76(10): 889-903.
- Holak G: Diagnosis and therapy of axial spondyloarthritis. DFP Literature Study 2021, www.pains.at/wp-content/uploads/SN-1-21-DFP-Diagnose-und-Therapie-der-axialen-Spondyloarthritis_komprimiert.pdf, (last accessed 07/14/2022).
- Rudwaleit M, et al: The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009; 60(3): 717-727.
- Axial spondyloarthritis including ankylosing spondylitis and early forms, AWMF Guidelines Register Number: 060/003, Development Stage: S3 Version: 2019.
- Garrett S, et al: A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994; 21(12): 2286-2291.
- van der Heijde D, et al: ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 2009; 68(12): 1811-1818.
- van der Heijde D, et al: Preliminary core sets for endpoints in ankylosing spondylitis. Assessments in Ankylosing Spondylitis Working Group. J Rheumatol 1997; 24(11): 2225-2229; 321.
- van der Heijde D, et al: Ankylosing spondylitis: plenary discussion and results of voting on selection of domains and some specific instruments. J Rheumatol 1999; 26(4): 1003-1005.
- “Targeted synthetic or biological DMARD in axial spondyloarthritis,” Prof. Désirée van der Heijde, MD, EULAR, June 04, 2022.
- Lindström U, et al: Anterior uveitis in patients with spondyloarthritis treated with secukinumab or tumor necrosis factor inhibitors in routine care: does the choice of biological therapy matter? Ann Rheum Dis 2021; 80(11): 1445-1452.
- Kivitz AJ, et al: Efficacy and Safety of Secukinumab 150 mg with and Without Loading Regimen in Ankylosing Spondylitis: 104-week Results from MEASURE 4 Study. Rheumatol Ther 2018; 5(2): 447-462.
- Deodhar A: COAST-W Study Group. Efficacy and Safety of Ixekizumab in the Treatment of Radiographic Axial Spondyloarthritis: Sixteen-Week Results From a Phase III Randomized, Double-Blind, Placebo-Controlled Trial in Patients With Prior Inadequate Response to or Intolerance of Tumor Necrosis Factor Inhibitors. Arthritis Rheumatol 2019; 71(4): 599-611.
- Kragstrup TW, et al: Waiting for JAK inhibitor safety data. RMD Open 2022 Feb; 8(1): e002236.
- Haller C: Axial Spondyloarthritis, www.kssg.ch/system/files/media_document/2022-05/Axiale%20Spondyloarthritis.pdf, (last accessed 07/14/2022).
HAUSARZT PRAXIS 2022; 17(8): 16-17











