Patients with idiopathic generalized epilepsy and children with Rolando’s epilepsy may not require an MRI scan at all. In contrast, high-resolution MRI diagnostics should definitely be pursued in focal epilepsies that cannot be treated with medication. The MRI field strength should be at least 1.5 Tesla, but ideally 3 Tesla. High spatial resolution with the MRI sequence with slice thicknesses ≤3 mm while maintaining an adequate signal-to-noise ratio is important to detect the often very small, subtle lesions. 3D sequences with isotropic voxel volumes not only allow multiplanar reformatting, they are also particularly suitable for automated post-processing (voxel-based analysis), in which additional, otherwise undetected dysplasias are found in about 5% of cases. The FLAIR sequence has the highest diagnostic value.
In general, the first step in epilepsy diagnosis is to distinguish whether the seizures are occasional or recurrent generalized or focal seizures. (Primary) generalized seizures originate from a specific point of a neuronal network involving both cerebral hemispheres with rapid spread. Point of onset and laterality are not constant from seizure to seizure, and an underlying epileptogenic lesion is absent.
Focal seizures originate from a neuronal network confined to one cerebral hemisphere, and an epileptogenic lesion is often found at this point. For each seizure type, the onset of individual seizures is constant and associated with preferred patterns of propagation that may involve the contralateral hemisphere. Focal seizures may progress without (formerly: simple-focal seizure) or with limitation of consciousness (formerly: complex-focal seizure) and may progress to generalized tonic, clonic, or tonic-clonic seizures (formerly: secondary generalized seizure) [1].
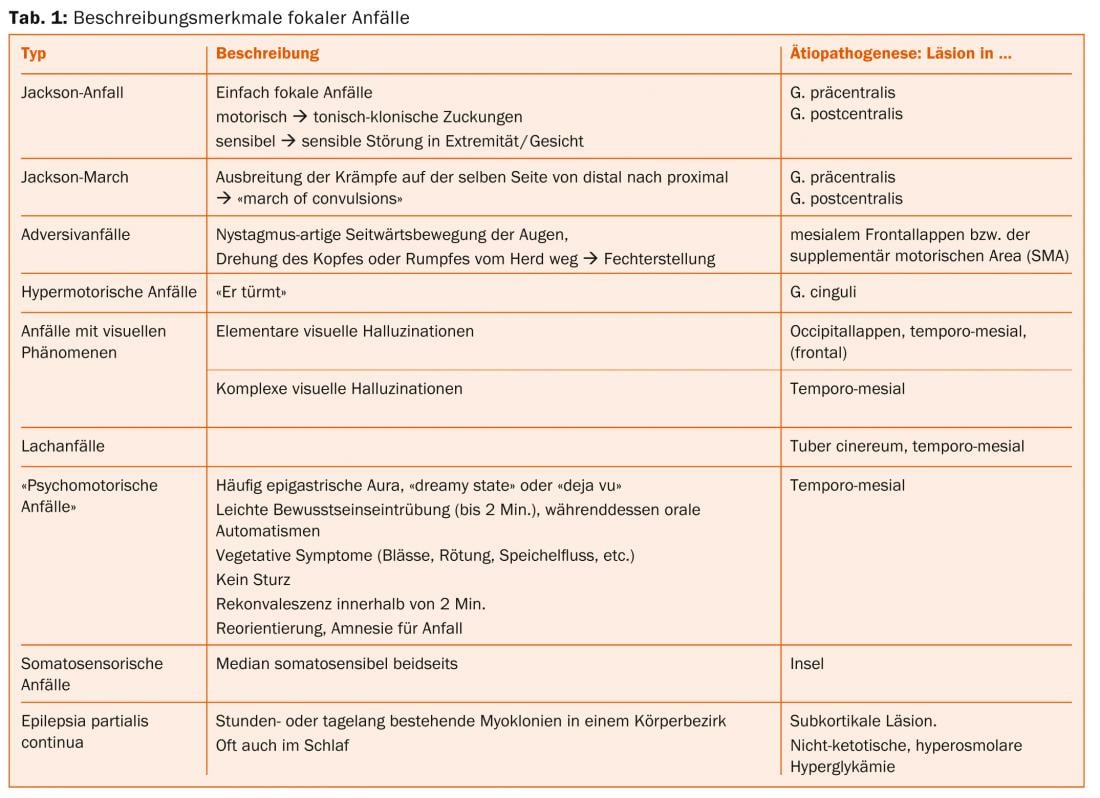
The neuroradiologist’s attention is focused on focal seizures [2]. Often, the so-called aura, i.e. the initial phase of a focal seizure remembered by the patient, and the clinical symptoms during the epileptic seizure indicate the seizure origin (Tab. 1).
However, the epileptogenic lesions in focal epilepsies are often overlooked [3]. There are three main reasons that they are overlooked:
- Epileptogenic lesions are often small and nonspace-occupying. They hardly change in the course of life and thus differ from a tumor or an infarct, which are recognized at the latest on the basis of their growth or shrinkage and demarcation on follow-up examinations.
- The neuroradiologist has no clinical information or is unable to classify the available clinical examinations. That is, he does not know whether he can find an epileptogenic lesion at all and, if so, in which region of the brain to look for it.
- The MR tomograms obtained are qualitatively inadequate in terms of anatomical orientation, spatial resolution, and signal-to-noise or contrast-to-noise ratio.
MRI protocol
The following points should be noted:
Field strength: The field strength should be at least 1.5 Tesla, ideally 3 Tesla. The signal-to-noise ratio at 3 Tesla is about 1.8 times higher than at 1.5 Tesla.
Orientation: Transverse (axial) slices are oriented either along a line through the commissura anterior and commissura posterior or along a line through the long axis of the hippocampi (“temporal angulation”). Coronal layers should always be oriented perpendicular to the long axis of the hippocampi (“temporal angulation”). To obtain a symmetrical representation of both cerebral hemispheres, it is important that the coronal plane is correctly aligned along the a.p. axis. Sequence planning is therefore optimally performed on a sagittal T1-weighted gradient echo sequence with thin slices and on an axial T2-weighted sequence. 3D sequences with isotropic voxels allow multiplanar reformatting so that non-exact angulations can be compensated posthoc.
Spatial resolution: epileptogenic lesions with the potential for epilepsy surgery are often as small as five to seven millimeters in diameter. Examples are small cavernomas, focal cortical dysplasias (FCD) of type 2b according to Palmini or small nodular periventricular heterotopias located in the depth of the sulcus; the latter, however, without prospect of seizure freedom after surgery. The probability of detecting an epileptogenic lesion depends on the contrast to the surrounding gray or white matter and on the voxel size of the sequences used. In order for a lesion not to be “masked” by partial volume effects, it should be three, in the best case two voxels in size. This means that the layer thickness of 2D sequences should not exceed three millimeters [4].
Contrasts: Spatial resolution and signal-to-noise ratio are inversely related. Both can be improved with a longer measurement time, but at the same time the probability of motion unsteadiness increases. A reasonable compromise must therefore be found between image quality and measurement time. Relatively often, therefore, MRI examinations are necessary in sedated patients or under intubation anesthesia. Comparing 2D and 3D sequences, 2D sequences have a better signal-to-noise ratio with generally higher slice thickness and better “in plane” resolution. Conversely, 3D images acquired sagittally can usually be reformatted accordingly and the lesion can thus be worked out.
Postprocessing (voxel-based morphometry): 3D sequences with isotropic, usually 1 mm3 voxels are used for postprocessing. In the procedure developed by H.J. Huppertz, based on the SPM software, T1-weighted images are first “normalized” to a standard brain and intensity corrected. This is followed by segmentation into gray matter, white matter and CSF and the creation of binary images (gray matter and white matter). From this, different mappings (“Thickness-, Extension-, Junction-Image”) can be calculated and differences can be “illuminated out” by subtraction with images of a control group. The quality of these images is highly dependent on the acquisition quality of both the individual patient and the not completely scanner-unspecific control group. Out-illuminated lesions may be false-positive lesions and in any case must be retrievable in the structural images [5].
Based on the above considerations, the Structural Imaging Commission of the German Section of the International League Against Epilepsy has recommended an MRI protocol consisting of the sequences described in Table 2 [4].
Additional contrast administration may be used for specification, not detection of an epileptogenic lesion. Image evaluation with voxel-based morphometry can be performed on an individual basis and is predominantly used by specialized neuroradiology departments in collaboration with epilepsy surgery centers.
Epileptogenic lesions
Resection results from large epilepsy surgery centers show that epileptogenic lesions can be differentiated into three broad groups: Hippocampal scleroses, glioneuronal tumors, focal cortical dysplasias [6]:
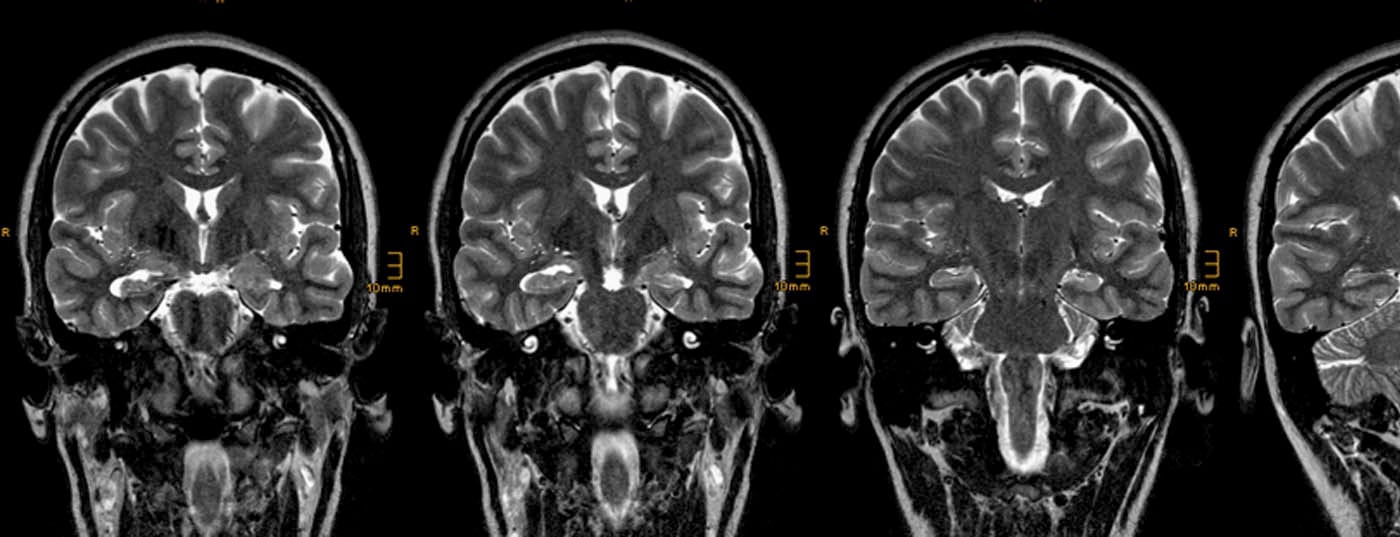
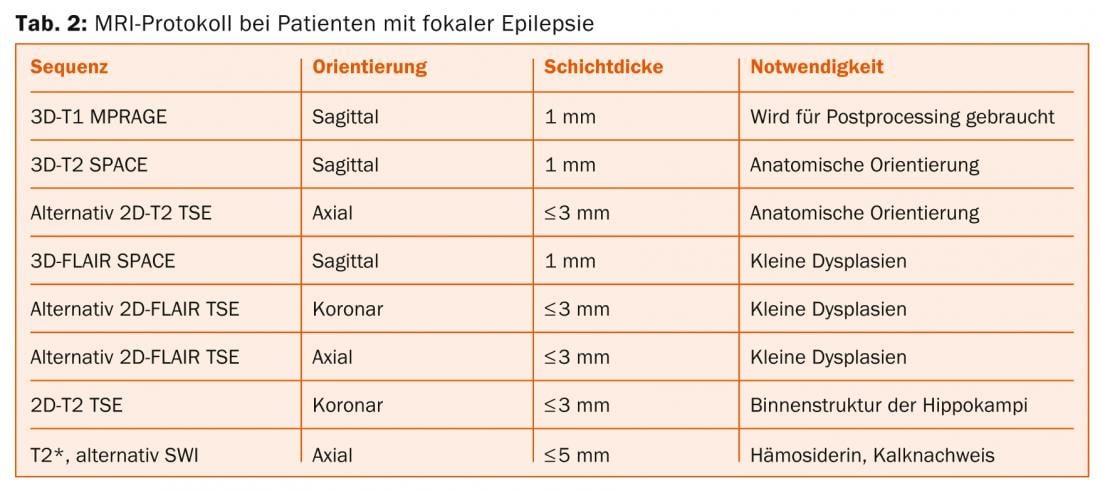
Hippocampal sclerosis: The MRI correlate of hippocampal sclerosis consists of atrophy and increased signal from the hippocampus on FLAIR and T2-weighted TSE images (Fig. 1) .
This pattern is best visualized on coronal temporal-angulated images approximately two to three millimeters thick. The following points should be taken into account:
- The layer through the hippocampal head is primarily considered because the relative volume of the hippocampus is greatest here, making lateral differences most readily apparent. In contrast, the neuropathological diagnosis of hippocampal sclerosis is made on coronal slices through the hippocampal body, because only on these slices is it possible to reliably assign the individual Sommerian sectors of the hippocampus.
- Comparing T2-weighted and FLAIR images, FLAIR images have a higher contrast-to-noise ratio. However, a disadvantage is that already healthy limbic structures show a higher signal intensity on FLAIR images, thus increasing the probability of false-positive diagnoses [7].
- Bilateral hippocampal sclerosis is found in up to 20% of patients; in these cases, determination of T2 relaxation time may be helpful.
- Hippocampal atrophy also causes the digitationes hippocampi, which are directed upward and are also best seen in the hippocampal head, to flatten, but this effect is also observed in age-related atrophy. Similarly, dilatation of the ventricular inferior horn is frequently observed, but the ventricular system is often asymmetric even in healthy individuals, so this sign is not very reliable.
- Hippocampal atrophy without signal abnormalities is extremely rare, described in less than 5% of histologically confirmed hippocampal sclerosis.
- Additional lesions besides hippocampal sclerosis are found in up to 20% of patients (“dual pathology”).
- Additional changes in the limbic system include atrophy of the amygdala, entorhinal cortex, ipsilateral corpus mamillare, ipsilateral fornix, and gray-white differentiation disorder of the anterior temporal lobe. The latter is also considered by some authors as focal cortical dysplasia and together with hippocampal sclerosis as a dual pathology.
Glioneuronal tumors: ganglioglioma and dysembryoplastic neuroepithelial tumor (DNT) have neuronal and glial elements (=glioneuronal tumors), are characterized by their location in the cortex and adjacent medullary camps, and typically cause epileptic seizures that cannot be treated with medication.
MRI correlates of ganglioglioma are cortical/subcortical location preferentially in the parahippocampal and temporo-occipital lateral gyrus. Classically, there is a combination of intracortical cyst(s), a circumscribed area of cortical/subcortical signal enhancement on FLAIR/T2-weighted images, and a contrast-receiving node. Calcification is found in one third of cases. If there is no contrast uptake (in approximately 50%), differentiation from dysplasia may be difficult. In these cases, the intracortical cyst(s) are the most seminal. Gangliogliomas are WHO°I tumors in 90% of cases and WHO°III tumors in 10% of cases. Indicative of WHO°III ganglioglioma are perifocal edema, extratemporal location, male sex, age >40 years, absence of epileptic seizures, and a gemistocytic tumor component [8].
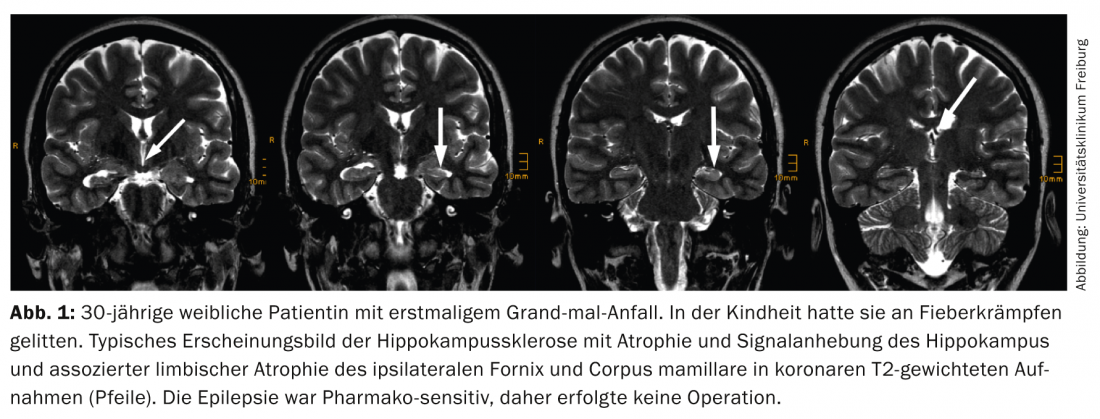
MRI correlates (of the simple variant) of DNT are multiple small cysts (Fig. 2) that correspond histologically to the glioneuronal element and are best detected on high-resolution T2-weighted TSE images.
In the complex variant, calcifications or even hemorrhages, which are separated from the glioneuronal element, are added. Annular contrast uptake within the glioneuronal element is sometimes seen; interestingly, this can disappear. Of clinical significance, DNTs are always WHO°I tumors and approximately 15% of DNTs are still confused with glial tumors (typically oligodendrogliomas) [9].
For a more detailed description also of the other tumors mentioned above, please refer to textbooks [10].
Astrocytic tumors with similar location and similar epileptogenicity are pilocytic astrocytoma and pleomorphic xanthoastrocytoma (PXA). The fourth edition of the WHO classification also included angiocentric glioma (angiocentric neuroepithelial tumor, ANET) under the category of other neuroepithelial tumors, which typically causes epileptic seizures and has histologic similarities to ependymoma [11]. The term “long term epilepsy associated tumors” (LEATs) is also commonly used for the above tumors [12].
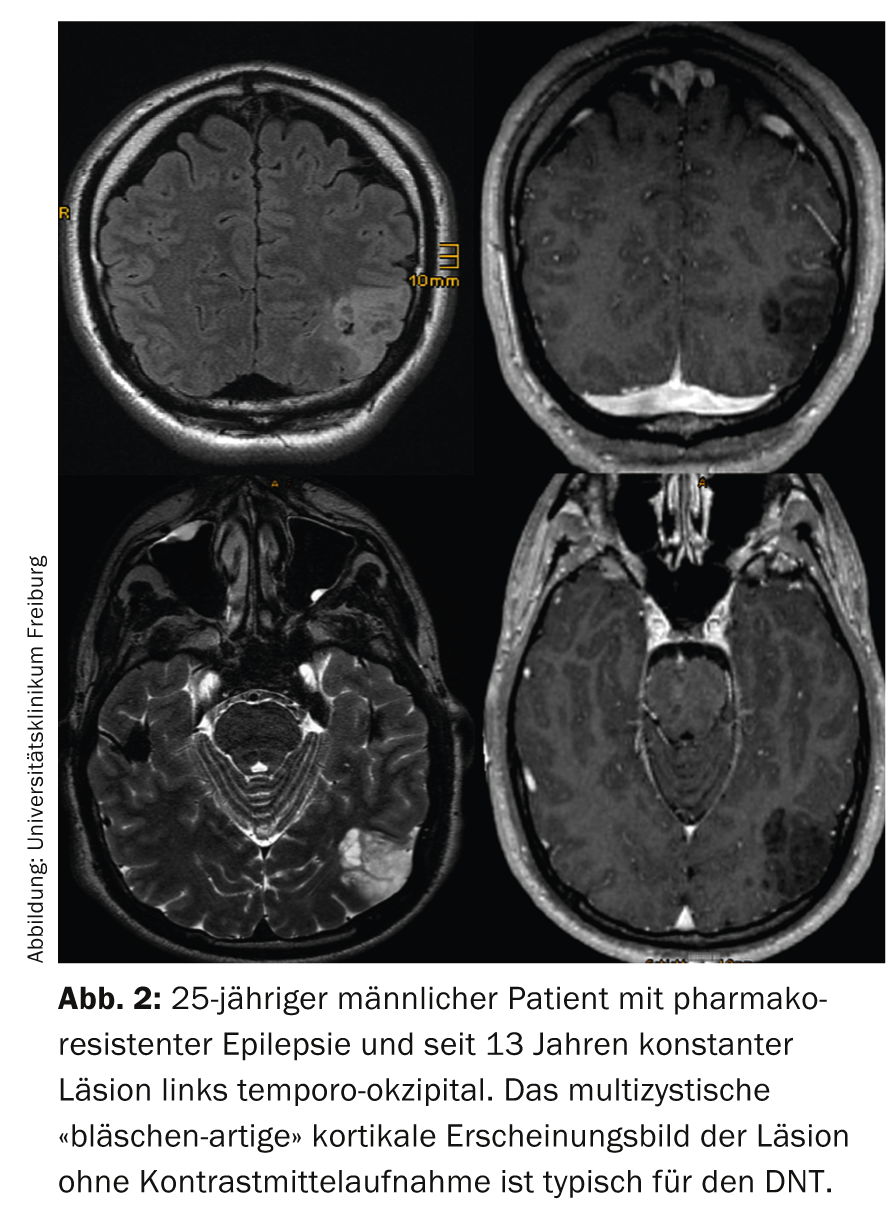
Focal cortical dysplasias (FCD): Classification and correlation to imaging is inconsistent between different epilepsy surgery centers; the only clearly defined dysplasia is FCD IIb characterized by balloon cells. On MRI, the altered cortex is isointense on T1-weighted images, iso- to slightly hyperintense on T2-weighted images, and slightly hyperintense on FLAIR images. In addition, there is a funnel-shaped, subcortical hyperintensity tapering toward the lateral ventricle and sometimes extending to the lateral ventricle, which is better seen on FLAIR- than on T2-weighted images (Fig. 3) and has led to the name transmantle dysplasia.
Lesions are usually solitary and neocortically located, most commonly affecting the frontal lobe (presumably due to lobe size). Multiple lesions are thought to be tuberous sclerosis and look for subependymal calcifications and giant cell astrocytoma. Lesions can vary in size: very small lesions are characteristically located in the sulcus and are easily missed on axial slice images. Because of the orientation, coronal and sagittal FLAIR images or a 3D dataset with appropriate reformatting are required here. Very large lesions may also involve the majority of one hemisphere at one time, and they are often not completely resectable, reducing the risk of postoperative seizure freedom below 50%.
In addition to the above entities, a variety of different epileptogenic lesions are found in patients with focal epilepsies, so that the proportion of MRI-negative examinations decreases – albeit slightly – over time [6,13]. For the physician and the patient, it is initially reassuring to have found the cause of the epileptic seizures. Especially in focal epilepsies that cannot be controlled with medication, this point and the fact that in suitable epileptogenic lesions about 70% of patients become seizure-free with surgery should be the reason for a targeted MRI examination and possible referral to an epilepsy surgery center.
PD Stephan Meckel, MD
Literature:
- Berg AT, et al: Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005-2009. Epilepsia 2010;1-10 Revised terminology and concepts for organization of seizures and epilepsies: report of the classification and terminology commission of the International League Against Epilepsy. Act Neurol 2010; 37: 120-130.
- Urbach H: Imaging of the epilepsies. Eur Radiol 2005; 15: 494-500.
- von Oertzen J, et al: Standard MRI is inadequate for patients with refractory focal epilepsy. J Neurol Neurosurg Psychiatry 2002; 73: 643-647.
- Wellmer J, et al: Proposal for a magnetic resonance imaging protocol for the detection of epileptogenic lesions at early outpatient stages. Epilepsia 2013 Oct 7. [Epub ahead of print].
- Huppertz HJ: Morphometric MRI Analysis. In: MRI in Epilepsy. Ed Urbach H. Springer Heidelberg, New York, Dordrecht, London 2013; 73-84.
- Bien CG, et al: Trends in presurgical evaluation and surgical treatment of epilepsy at one center from 1988-2009. J Neurol Neurosurg Psychiatry 2013 Jan; 84(1): 54-61.
- Hirai T, et al: Limbic lobe of the human brain: Evaluation with Turbo Fluid-attenuated Inversion-Recovery MR Imaging. Radiology 2000; 215: 470-475.
- Majores M, et al: Tumor recurrence and malignant progression of gangliomas. Cancer 2008; 113: 3355-3363.
- Campos AR, et al: Simple and Complex Dysembryoplastic Neuroepithelial Tumors (DNT): clinical profile, MRI and histopathology. Neuroradiology 2009; 51: 433-439.
- Urbach H: Epilepsy associated tumors and tumor-like lesons. In: MRI in Epilepsy. Ed Urbach H. Springer Heidelberg, New York, Dordrecht, London 2013; 109-124.
- Louis DN, et al: The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97-109.
- Luyken C, et al: The Spectrum of Long-term associated Tumors: Long-term Seizure and Tumor outcome and Neurosurgical aspects. Epilepsia 2003; 44: 822-830.
- Bien CG, et al: Characteristics and surgical outcome of patients with refractory MRI-negative epilepsies. Arch Neurol 2009; 66: 1491-1499.
- Wagner J, et al: Morphometric MRI analysis improves detection of focal cortical dysplasia type.II. Brain 2011 Oct; 134(10): 2844-2854.
InFo NEUROLOGY & PSYCHIATRY 2014; 12(4): 4-9.