At the 20th EHA Congress in Vienna, experts discussed the therapeutic situation in myelodysplastic syndromes. How does the frequently prescribed active ingredient azacitidine perform in practice? Data from a Dutch registry were presented. Furthermore, the treatment of patients who do not (or no longer) respond to erythropoiesis-stimulating substances was discussed. In this context, evaluations on quality of life from the MDS-005 study with lenalidomide and innovative concepts such as the new active substance sotatercept were presented.
For high-risk patients with myelodysplastic syndrome (MDS) who are not eligible for transplantation, azacitidine (Vidaza®) has been available for some time. Real-life studies examining the use and success of this agent in clinical practice and comparing it to conventional regimens remain scarce. Using the Dutch PHAROS MDS registry, researchers presented results from 515 MDS patients diagnosed between 2008 and 2011 at the EHA Congress in Vienna. None of the patients were eligible for transplantation. 29% were at low risk and 22% at high risk according to IPSS, a validated prognostic system used in risk assessment of MDS patients. In the remaining 49%, no IPSS score could be defined due to lack of cytogenetic findings.
Of interest for this specific analysis were the 113 patients with high-risk MDS. Of these, 65 received azacitidine, 32 received supportive care as possible (including treatment with growth factors), and 16 underwent intensive chemotherapy. Patients in the latter group were older and had worse ECOG performance status (≥2) than the others. The median age here was 77 years, in the azacitidine group 74 years, and in the chemotherapy group 66 years.
- Azacitidine and chemotherapy were administered for a median of seven and two cycles, respectively. In contrast, supportive care was provided for only 3.9 months.
- After a median follow-up of 14.7 months, overall survival was 17.6 months in the azacitidine group versus 9.1 months in the supportive group. This results in a significant 40% reduction in the risk of death. With intensive chemotherapy, patients survived a median of 19 months (HR 0.73; p=0.321).
- The 1-year survival rates were 72%, 38%, and 75%. The difference between the supportive care group and the azacitidine group was significant (p=0.005).
- Complete response was 12%, 0%, and 38%, and partial response was 3%, 0%, and 0%.
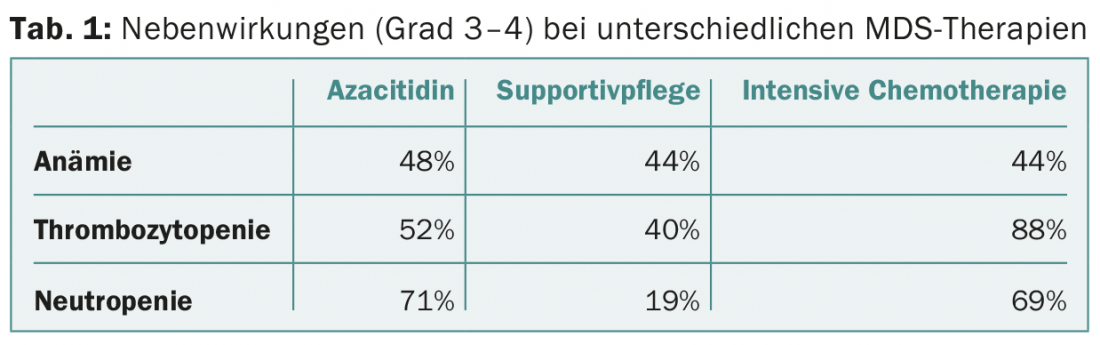
- Hematologic side effects of grade 3-4 are shown in Table 1.
Valuable addition to controlled data
The study authors concluded that azacitidine prolonged survival over supportive care but not chemotherapy in this population-based study. In the long term, the mortality benefit in this real-life cohort was lower than in the controlled phase III trial called AZA-001 [1] – a result that the researchers say should be interpreted cautiously because of the uncontrolled nature of their study, but is nonetheless a valuable addition to the clinically randomized data. Conclusions should also be made cautiously because of the limited number of patients. The hematologic adverse event profile and overall response rates were comparable to known data from AZA-001.
Evaluation of quality of life in MDS-005
A major characteristic of MDS is anemia, and when erythropoiesis-stimulating agents do not work or no longer work, other strategies must be found to reduce the need for transfusion. MDS-005 is a phase III trial that was presented at the ASH Congress in December 2014 [2]. It showed that significantly more (initially transfusion-dependent) MDS patients achieved transfusion independence – i.e., no need for red cell concentrates for at least 56 consecutive days – with lenalidomide than with placebo (26.9% vs. 2.5%; p< 0,001). This was the primary endpoint of the study.
A prespecified secondary endpoint was health-related quality of life, assessed with the European Organization for Research and Treatment of Cancer QLQ-C30 questionnaire. Quality of life is usually severely compromised in MDS patients with transfusion-dependent anemia.Participants in the MDS-005 study did not respond (or no longer responded) to erythropoiesis-stimulating agents and had “low” or “intermediate 1” risk disease without del(5q) according to the IPSS – a population for which there are few treatment options. 160 received lenaliodmide and 79 placebo. Five sections of the QLQ-C30 questionnaire were defined as clinically relevant and selected in advance for analysis: Fatigue, Dyspnea, Physical/Emotional Functioning, and Overall Quality of Life.
Twelve weeks after randomization, the quality of life scores of the two treatment arms did not yet differ in the relevant five points. However, from week 24, lenalidomide performed significantly better in terms of emotional functionality (p=0.047). Patients who responded to lenalidomide and were treated with it through week 24 showed increasing benefit in all areas as the duration of therapy progressed. In a post-hoc analysis, transfusion independence was significantly associated with improvements in quality of life. For those individuals who achieve transfusion independence after failure of erythropoiesis-stimulating agents on lenalidomide, quality of life also increases, the study investigators concluded.
Further drugs in the pipeline
For heavily pretreated patients, other agents are currently under investigation. One of these is sotatercept (ACE-011), the first representative of a new class of drugs that bind activins. This results in an erythropoiesis-stimulating effect [3]. In a phase II trial involving 59 patients with MDS (risk “low”/”intermediate 1”) and anemia, the compound has now been shown to provide clinical benefit. All subjects failed to respond or had a very low probability of response to erythropoiesis-stimulating agents. Overall, 95% of patients had already been treated with erythropoiesis-stimulating agents (in addition to various other MDS therapies such as lenalidomide or hypomethylation drugs). The median age was 71 years. Hematologic improvement (HI-E; modified International Working Group 2006 criteria) was considered the primary endpoint, with secondary endpoints including transfusion independence for at least eight weeks. In the two months prior to initiation of therapy, patients had received a median of six red cell concentrates. They were therefore transfusion-dependent – the majority of patients even had a high transfusion requirement.
The primary endpoint of relevant hematologic improvement was met by 43% with sotatercept. The highest rate (67%) was found in the group receiving a dose of 0.3 mg/kg sotatercept every three weeks. In the high transfusion burden group (≥4 concentrates in eight weeks), transfusion independence was achieved in 13% for at least eight weeks. In the much smaller group with a low transfusion load (<4 units), this was true for 63%.
The substance was well tolerated. A total of four patients dropped out due to therapy-associated adverse events. At least one grade 3-4 adverse effect was found in 31%, of which 5% were potentially drug-related (pain in extremities, hypertension, acute myeloid leukemia). All serious adverse events occurred in the group that received sotatercept at the dose of 0.5 mg/kg.
In this heavily pretreated population, sotatercept showed encouraging activity and a good safety profile, the study authors concluded.
Source: 20th EHA Congress, June 11-14, 2015, Vienna.
Literature:
- Fenaux P, et al: Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009 Mar; 10(3): 223-232.
- Santini V, et al: Efficacy and Safety of Lenalidomide (LEN) Versus Placebo (PBO) in RBC-Transfusion Dependent (TD) Patients (Pts) with IPSS Low/Intermediate (Int-1)-Risk Myelodysplastic Syndromes (MDS) without Del(5q) and Unresponsive or Refractory to Erythropoiesis-Stimulating Agents (ESAs): Results from a Randomized Phase 3 Study (CC-5013-MDS-005). Blood 2014; 124: abstract 409.
- Carrancio S, et al: An activin receptor IIA ligand trap promotes erythropoiesis resulting in a rapid induction of red blood cells and haemoglobin. Br J Haematol 2014 Jun; 165(6): 870-882.
InFo ONCOLOGY & HEMATOLOGY 2015; 3(8): 30-31.