Neoadjuvant chemotherapy is always possible when adjuvant chemotherapy is also indicated. During neoadjuvant chemotherapy, treatment response should be documented so that treatment can be modified if necessary. Neoadjuvant chemotherapy allows surgery within new limits. Neoadjuvant chemotherapy can buy time without delaying therapy.
Chemotherapy for breast carcinoma is increasingly being given before surgery as neoadjuvant therapy throughout Europe. A trend in this direction can also be seen in Switzerland. This article will focus on the indications for neoadjuvant chemotherapy and its benefits.
Breast conservation instead of mastectomy
Even today, mastectomy is necessary in approximately 25% of patients with breast carcinoma because the tumor size in relation to breast size does not allow for any other procedure that is oncologically safe. In these cases, neoadjuvant chemotherapy may reduce the extent of surgery if response is good, as surgery can be performed within new limits without affecting overall survival or locoregional control [1].
Prior to initiation of therapy, the tumor should be marked with a clip so that the tumor bed can be localized during surgery in case of complete remission after neoadjuvant chemotherapy.
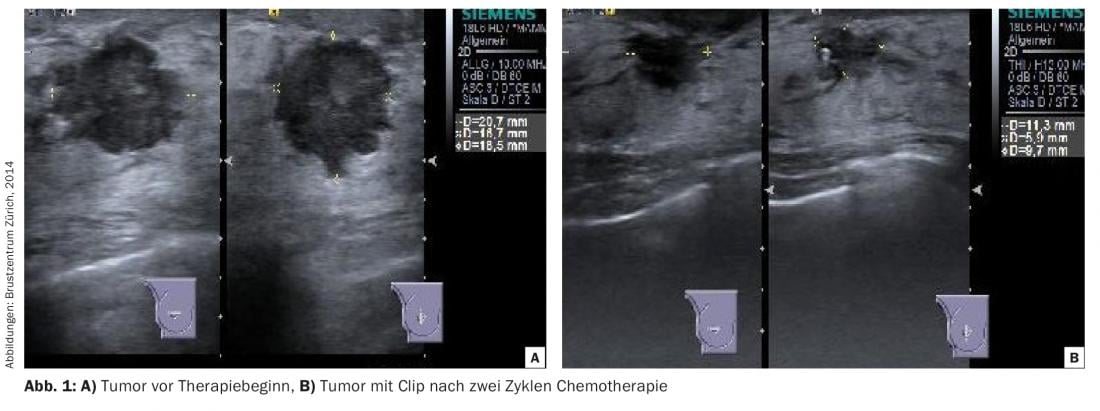
During therapy, the response should be documented (“response monitoring”). Ultrasound is usually best suited for this purpose, even though delineation of the residual tumor can be difficult due to tissue fibrosis (Fig. 1) . In the case of lobular or multicentric carcinomas, which are often occult in their overall extent in images by mammography and ultrasound, progress controls by MRI may be necessary.
Axillary downstaging
In clinically negative axilla, sentinel lymphonodectomy is usually performed before (possibly with implantation of a port-à-cath), but sometimes also after neoadjuvant chemotherapy. If the axilla is clinically positive, histologic confirmation of the metastasis is first performed by punch biopsy. The subsequent procedure has been defined at the SENTINA and ACOSOG Z1071 trial in the 2015 St. Gallen Consensus Conference.
After downstaging of an initially affected axilla, sentinel lymphonodectomy is an adequate procedure after neoadjuvant chemotherapy. Sentinel labeling should be done with patent blue in addition to the radiocolloid. The reliability of sentinel lymphonodectomy in this situation depends on the number of lymph nodes removed. Only with three tumor-free lymph nodes removed is the false-negative rate (FNR) comparable to the FNR with primary surgery, and axillary lymphonodectomy can be omitted. On the other hand, if one or two sentinel lymph nodes are affected after neoadjuvant chemotherapy, axillary lymphonodectomy should be performed [2–4].
Inflammatory breast carcinoma
A characteristic feature of inflammatory breast carcinoma is its spread along the lymphatic pathways, which means that the potential for metastasis is high. Neoadjuvant chemotherapy is mandatory in this situation. Response to therapy is an important factor in the patient’s prognosis. Regarding surgery, there has been no change to date, and mastectomy remains the gold standard even in cases of clinical complete remission.
In vivo testing
During therapy, imaging, usually sonographic, controls are performed to document tumor response. If the tumor shows little or no response to therapy, the therapy regimen can be changed (“response guided treatment”), which improves the prognosis for patients with less aggressive carcinomas [5].
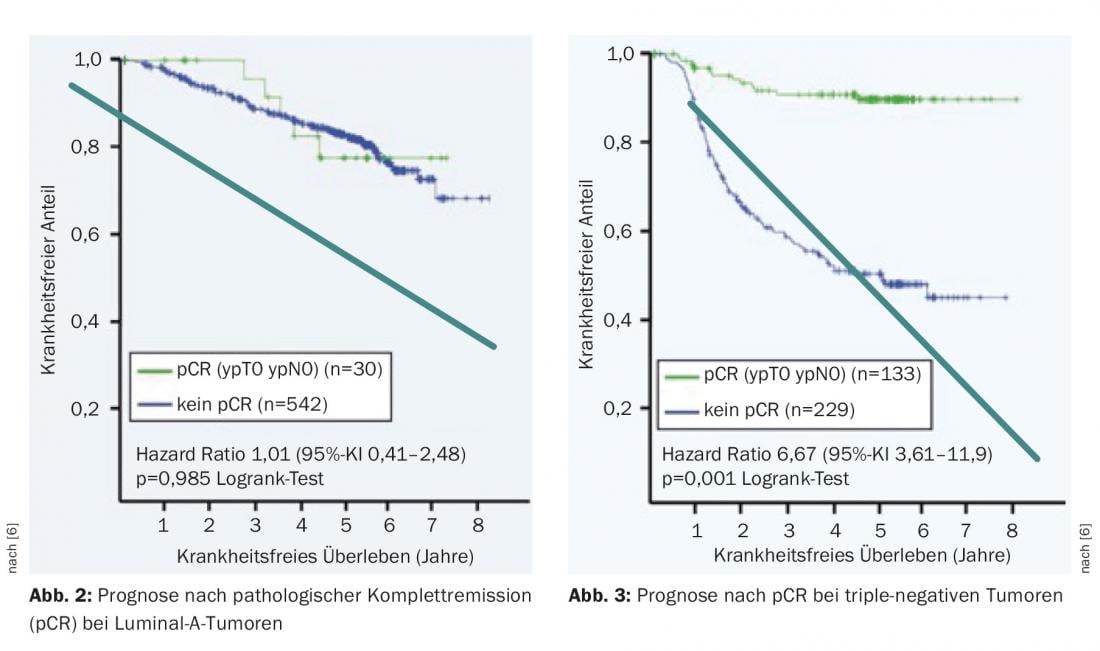
In tumor response, a distinction is made between partial and complete remission. Pathologic complete remission (pCR) is an important prognostic factor in the more aggressive tumor types. In patients with hormone receptor-positive, Her2-negative carcinomas (Luminal-A), pCR is prognostically insignificant (Fig. 2) . In contrast, patients with triple-negative tumors have a chance of a significantly better outcome in terms of disease-free survival (Fig. 3) . In addition, it is motivating for the patient if she can observe how the tumor is getting smaller. This psychological effect leads to improved compliance to endure the stressful therapy.
Time saving
Some situations require a window of time before surgery in which further clarification can be made. Increasingly, time-consuming genetic testing with a possible consequence for the surgical procedure (e.g., mastectomy instead of breast-conserving therapy, bilateral mastectomy) is performed. It usually takes three weeks until the result of a genetic test is available. With primary planned surgery, there is a significant delay in therapy.
The potential consequences of a positive genetic test must be well considered and represent a not inconsiderable stress factor for the patient, so a window of three to four months before surgery is valuable. Furthermore, if mastectomy is planned (e.g., for multicentric or inflammatory carcinoma), the time gained can be used to evaluate breast reconstruction.
Conclusion
In summary, chemotherapy should always be considered before surgery if chemotherapy is also indicated postoperatively. The advantages are the determination and monitoring of response with possible therapy adjustment in the absence of remission, the reduction of tumor size with possible breast preservation if mastectomy is planned in advance, and the time saved for preoperative investigations (e.g. genetic testing). In inflammatory carcinoma, neoadjuvant chemotherapy is mandatory.
However, despite the aforementioned advantages, chemotherapy in the neoadjuvant setting does not confer a survival advantage over adjuvant therapy. Future studies should further evaluate the value of neoadjuvant therapy.
Literature:
- Mieog JS: Neoadjuvant chemotherapy for early breast cancer. Expert Opin Pharmacother 2009; 10(9): 1423-1434.
- Boughey JC, et al: Sentinel lymph node surgery after neoadjuvant chemotherapy in pathients with node positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013; 310: 1455-1461.
- Kuehn T, et al: Sentinel-lymph-node biopsy with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective multicenter cohort study. Lancet Oncol 2013; 14: 609-618.
- Boughey JC, et al: Methods impacting the false negative rate of sentinel lymph node surgery in patients presenting with node positive breast cancer (T0-T4, N1-2) who receive neoadjuvant chemotherapy – Results from a prospective trial – ACOSOG Z1071 (Alliance). San Antonio Breast Cancer Symposium 2014, P2-01-02.
- von Minckwitz, G: Update on neoadjuvant/preoperative therapy of breast cancer: experiences from the German Breast Group. Curr Opin Obstet Gynecol 2013; 25(1): 66-73.
- von Minckwitz G, et al: Neoadjuvant chemotherapy adapted by interim response improves overall survival of primary breast cancer patients: results of the GeparTrio trial. Cancer Res 2011; 71(Suppl 24): 103s.
InFo ONCOLOGY & HEMATOLOGY 2016; 4(1): 16-18.