The field of inflammatory skin diseases is constantly evolving. Updates on current standard treatments, innovative therapy options and artificial intelligence were presented at two accredited live webinars entitled “New Horizons in Dermatology” in June 2024. Below you will find a summary of the most important concepts presented.
Two live webinars entitled “New horizons in dermatology” took place on June 13, 2024 (for listeners from French-speaking Switzerland) and June 27, 2024 (for listeners from German-speaking Switzerland). The two interdisciplinary training courses focused on various inflammatory skin diseases. Prof. Dr. med. Nikhil Yawalkar (Inselspital Bern) and Prof. Dr. med. Curdin Conrad (CHUV Lausanne) dealt with psoriasis (PsO), Prof. Dr. med. et. Dr. phil. nat. Christoph Schlapbach (Inselspital Bern) and Dr. Basile Darbellay (Orsières) with atopic dermatitis (AD) and Prof. Dr. Falk Bechara (Ruhr University Bochum) with hidradenitis suppurativa (HS). Finally, the webinars were rounded off with an excursion into artificial intelligence (AI) and its significance for the dermatology of the future by Dr. Ludovic Amruthalingam (Lucerne University of Applied Sciences and Arts).
More than just the skin: Holistic PsO management
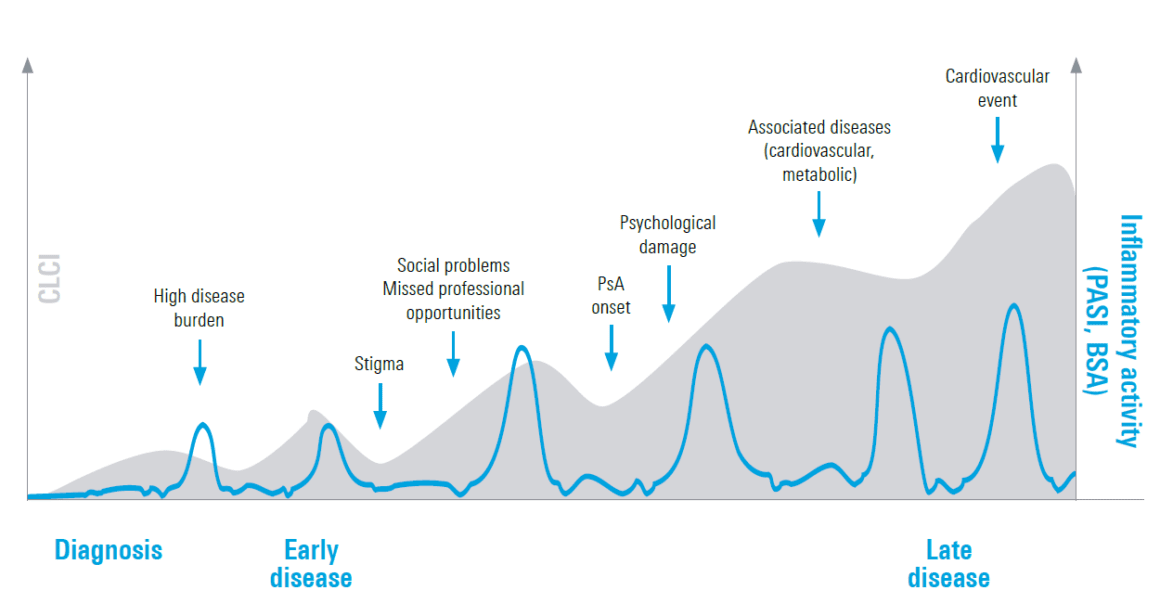
PsO is a heterogeneous disease with different manifestations [1]. In addition, even moderate to severe PsO has a high impact on quality of life, partly because the disease burden can be exacerbated by other comorbidities, according to the speakers at both events [1]. The disease burden can accumulate over a lifetime – this is also referred to ascumulative life course impairment (CLCI) [2, 3]. In this concept, the burden of physical and psychological comorbidities adds up over time and causes lifelong impairment (Fig. 1) [2, 3]. Early and adequate treatment could therefore be of great importance, said the two speakers. Various studies also indicate that patients with a short disease duration (≤2 years) respond better to systemic treatment than patients with a long disease duration (>2 years) [4, 5]. Current real-world data with SKYRIZI® (risankizumab) show that patients with a short disease duration are more likely to achieve a PASI 100 (80.4 % after one year, 93.3 % after three years) than patients with a longer disease duration (61.7 % after one year, 67.5 % after three years) [5]. According to the speakers, this shows that the disease should be treated optimally in the right “window of opportunity” and could potentially mitigate or even prevent the further course of the disease and/or comorbidities.
The challenge of AD: therapeutic goals and treatment options
AD is also a complex disease. At the beginning of his presentation, Dr. Basile Darbellay summarized the most important steps in AD pathogenesis: Penetration of healthy skin, activation of the innate immune system and subsequent cytokine production by Th2 cells [6]. Cytokines such as interleukin (IL)-4, IL-5, IL-13, IL-31 and IL-33 play a central role in the pathogenesis [6]. The therapeutic options for AD now range from topical to systemic treatments, with Janus kinase (JAK) inhibitors in particular having the potential to inhibit a number of the signaling pathways essential for AD [6]. Data from a large network meta-analysis (NMA) with 11 studies and over 6000 patients show that the JAKi RINVOQ® (upadacitinib) at 15 mg/day can achieve a high degree of skin clearance and itch relief in moderate to severe AD and is one of the most effective systemic therapies for AD over 12-16 weeks [7]. Later in the webinar, Prof. Schlapbach discussed the current AHEAD recommendations and emphasized the importance of shared decision-making and the Minimal Disease Activity (MDA) concept [8]. Data from the Measure Up 1 and 2 studies show that almost half of patients treated with RINVOQ® achieve MDA after 16 weeks and can maintain it for up to 52 weeks, compared to 6% on placebo [9].
Management of the HS: Quo vadis?
“HS is currently experiencing a real hype,” said Prof. Falk Bechara at the beginning of his lecture. The disease has a massive impact on the physical, social and economic aspects of patients [10-13]. HS is a relapsing-remitting disease, but in contrast to PsO, not only the comorbidity and restrictions in quality of life accumulate, but also the irreversible transformation of abscesses into tissue damage, according to Prof. Bechara. This makes it all the more important to find the right “window of opportunity” for treatment, i.e. to start treatment in the early phase of HS [14]. The updated German guidelines also distinguish for the first time between active and non-active HS [15]. For example, inflammation in active HS is now measured using the IHS method, as opposed to Hurley grading in non-active HS [15]. In active HS, TNF-α inhibitors such as HUMIRA® (adalimumab) and anti-IL-17 continue to receive the highest recommendation [15]. In addition, many clinical trials on JAK inhibitors and biologics are currently underway with the aim of further improving patients’ quality of life [16].
Dermatology of the future: the potential of AI
At the end of the two training sessions, Dr. Ludovic Amruthalingam rounded off the program with an overview of the potential of AI in dermatology. He explained the basics and methods of AI, machine learning (ML) and deep learning (DL), and pointed out the advantages of using the term “augmented intelligence” instead of “artificial intelligence“. According to Dr. Amruthalingam, DL models in particular can be used throughout the entire patient journey, from examination to diagnosis to treatment selection, providing objective and precise support. For example, precise counting of lesions in pustular PsO can significantly improve the granularity and objectivity of severity assessment. While manual counting is hardly feasible in everyday clinical practice, DL models can perform this task in just a few seconds [17]. In addition, there are already initial approaches to using large language models such as Meditron for clinical decision support and patient communication [18]. Finally, Dr. Amruthalingam sums up: “AI will not replace dermatologists, but certainly all dermatologists will soon use AI as an additional tool”.
Conclusion
The therapeutic field for inflammatory skin diseases is constantly changing. The diseases often have far-reaching effects on the lives of those affected, which can extend far beyond the skin. The underlying inflammation is crucial – effective, early treatment can therefore have a positive effect in reducing the burden of comorbidities and improving quality of life.
Literature
1 Griffiths, C.E.M., et al, Psoriasis. Lancet, 2021. 397(10281): p. 1301-1315.
2 Linder, M.D., et al, Psoriasis – The Life Course Approach. Acta Derm Venereol, 2016. 96(217): p. 102-8.
3 Warren, R.B., C.E. Kleyn, and W.P. Gulliver, Cumulative life course impairment in psoriasis: patient perception of disease-related impairment throughout the life course. Br J Dermatol, 2011. 164 Suppl 1: p. 1-14.
4 Schakel, K., et al, Early disease intervention with guselkumab in psoriasis leads to a higher rate of stable complete skin clearance (‘clinical super response’): Week 28 results from the ongoing phase IIIb randomized, double-blind, parallel-group, GUIDE study. J Eur Acad Dermatol Venereol, 2023. 37(10): p. 2016-2027.
5 Gargiulo, L., et al, A risankizumab super responder profile identified by long-term real-life observation-IL PSO (ITALIAN LANDSCAPE PSORIASIS). J Eur Acad Dermatol Venereol, 2024. 38(1): p. e113-e116.
6 Weidinger, S., et al, Atopic dermatitis. Nat Rev Dis Primers, 2018. 4(1): p. 1.
7 Silverberg, J.I., et al, Comparative Efficacy of Targeted Systemic Therapies for Moderate to Severe Atopic Dermatitis without Topical Corticosteroids: Systematic Review and Network Meta-analysis. Dermatol Ther (Heidelberg), 2022. 12(5): p. 1181-1196.
8 Silverberg, J., et al. Combining treat-to-target principles and shared decision-making: International expert consensus-based recommendations with a novel concept for minimal disease activity criteria in atopic dermatitis. J Eur Acad Dermatol Venereol, 2024. Epub ahead of print.
9 Silverberg J, et al. Treatment With Upadacitinib Increases the Achievement of Minimal Disease Activity Among Patients With Moderate-to-Severe Atopic Dermatitis: Results From Phase 3 Studies (Measure Up 1 and Measure Up 2). Presented at AAD, San Diego, March 8-12, 2024. 53959.
10 Dufour, D.N., L. Emtestam, and G.B. Jemec, Hidradenitis suppurativa: a common and burdensome, yet under-recognized, inflammatory skin disease. Postgrad Med J, 2014. 90(1062): p. 216-21; quiz 220.
11 Matusiak, L., A. Bieniek, and J.C. Szepietowski, Hidradenitis suppurativa markedly decreases quality of life and professional activity. J Am Acad Dermatol, 2010. 62(4): p. 706-8, 708 e1.
12 Abu Rached, N. et al. Diabetes remission associated with optimized treatment of hidradenitis suppurativa. J Dtsch Dermatol Ges, 2024. Online ahead of print.
13 Abu Rached N., et al. A state-of-the-art systematic review of cancer in hidradenitis suppurativa. Ann Med. 2024 Dec;56(1):2382372.
14 Marzano, A.V., et al, Evidence for a ‘window of opportunity’ in hidradenitis suppurativa treated with adalimumab: a retrospective, real-life multicentre cohort study. Br J Dermatol, 2021. 184(1): p. 133-140.
15 Zouboulis, C.C., et al, S2k guideline for the treatment of hidradenitis suppurativa / acne inversa – Short version. J Dtsch Dermatol Ges, 2024. 22(6): p. 868-889.
16. current clinical studies on hidradenitis suppurativa at www.clinicaltrials.gov.
17 Amruthalingam, L., et al, Quantification of Efflorescences in Pustular Psoriasis Using Deep Learning. Healthc Inform Res, 2022. 28(3): p. 222-230.
18 Chen Z, et al. MEDITRON: Open Medical Foundation Models Adapted for Clinical Practice. 2024. preprint available at: 10.21203/rs.3.rs-4139743/v1. .
19 Kimball, A.B., et al, Psoriasis: is the impairment to a patient’s life cumulative? J Eur Acad Dermatol Venereol, 2010. 24(9): p. 989-1004.
20 Ros, S., L. Puig, and J.M. Carrascosa, Cumulative life course impairment: the imprint of psoriasis on the patient’s life. Actas Dermosifiliogr, 2014. 105(2): p. 128-34.
21 Pariente, B., et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis, 2011.17:p.1415-22.
The references can be requested by specialists at medinfo.ch@abbvie.com.
Report: Dr. sc. nat. Stefanie Jovanovic
This article was produced with the financial support of AbbVie AG, Alte Steinhauserstrasse 14, 6330 Cham.
CH-ABBV-240083 08/2024
This article has been released in German.