Malignant tumors of the gastrointestinal tract are common, and the need for additional therapeutic options is high. At this year’s ASCO Annual Meeting, exciting new study results were presented for colorectal carcinomas as well as for carcinomas of the esophagus, gastroesophageal junction, and stomach. These could soon change everyday clinical practice.
Malignant tumors of the gastrointestinal tract are common. In the United States, 338 090 new cases (approximately 20% of all expected newly diagnosed malignancies) and 169 280 deaths (approximately 30% of all deaths caused by malignancies) are expected this year [1]. According to the Cancer Registry of Switzerland, 22 505 people in Switzerland developed colorectal cancer (CRC) during the observation period from 2013-2017 and there were 8939 deaths caused by this disease. During the same period, 5009 of the 7727 individuals who had carcinomas of the esophagus (Ö), gastroesophageal junction (gastroesophageal junction/GEJ), and stomach died [2]. These figures highlight the need for further therapeutic options for the treatment of tumor diseases of these organ systems.
New trial results were presented at the American Society of Oncology ( ASCO) Annual Meeting in June 2021 for colorectal carcinomas and for carcinomas of the esophagus, gastroesophageal junction, and stomach. The following is a review of current treatment standards and recently presented scientific advances of particular interest.
Colon carcinomas
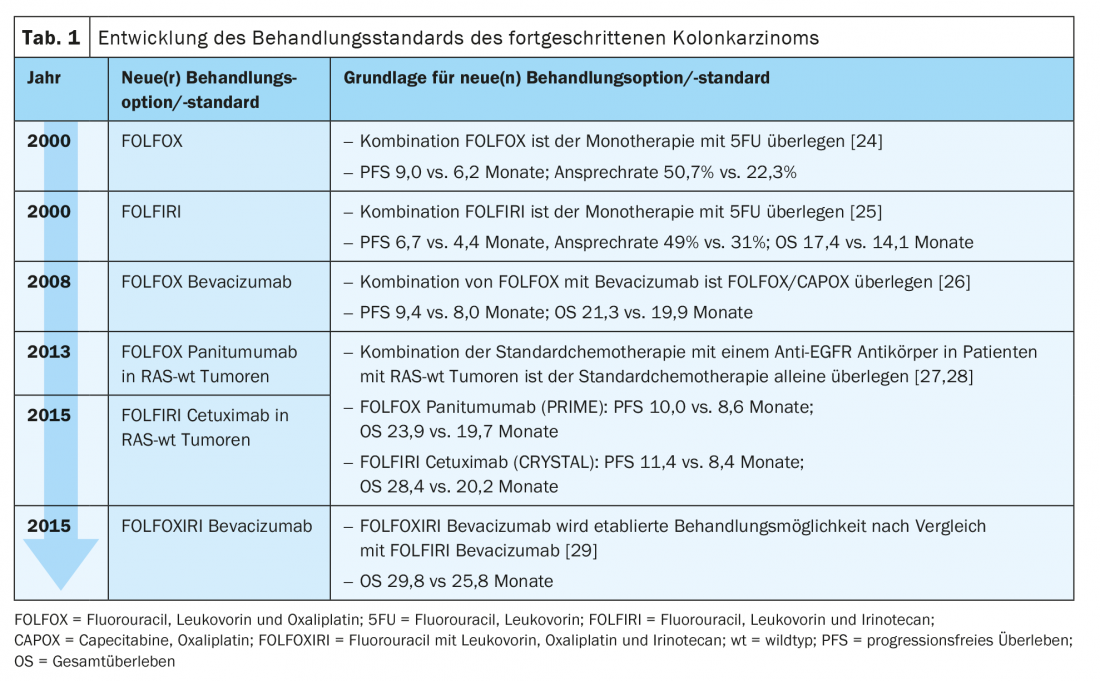
Nowadays, the standard treatment of advanced colon carcinoma in the first line of treatment consists of a combination of two to three chemotherapeutic agents with an antibody (Ab) against the Epidermal Growth Factor Receptor (EGFR) or against the Vascular Endothelial Growth Factor (VEGF) (Tab. 1) .
With the increasing use of molecular pathology in clinical practice, the analysis of microsatellite instability (MSI) has also become possible as an approximation of any defects present in the DNA mismatch repair system. Even if the frequency of MSI in CRC is stage-dependent and it is more often found in early stages (I/II: approx. 20%; III: approx. 12%; IV: 4-5%) [3], MSI is nevertheless also of interest in advanced tumor stages, especially in the context of immunotherapeutic treatment options. Le et al. showed back in 2015 that the presence of mismatch repair deficiency (MMR-d) in carcinomas of different tissue of origin is associated with a higher response rate ( RR), longer progression-free survival (PFS), and improved overall survival ( OS) when treated with pembrolizumab, an anti-PD-1 antibody. The collective of this study was composed of a large proportion of CRC patients [4]. Based on this, the use of the monoclonal anti-PD-1 Ab pembrolizumab and nivolumab – also in combination with the anti-CTLA4 Ab ipilimumab – in patients with advanced colon carcinoma is currently being investigated.
Keynote-177
The phase III KEYNOTE-177 trial compared the use of pembrolizumab (trade name KEYTRUDA®; manufacturer Merck/MSD) as a first-line treatment for advanced and metastatic CRC with a high level of MSI (MSI-H) with standard chemotherapy. 307 patients were randomized to an intervention arm with pembrolizumab 200 mg three weeks and a comparison arm with standard fluorouracil-based chemotherapy +/- bevacizumab or cetuximab. The primary endpoints were OS and PFS. The second interim analysis after a median observation period of 32.4 months already showed a clearly superior mPFS for immunotherapy (16.5 vs. 8.2 months). The overall response rate ( ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST) was also significantly higher in the pembrolizumab group (43.8% vs. 33.1%). In addition, there were significantly fewer severe treatment-related adverse events ( TRAEs) in the intervention group (22% TRAEs ≥grade3 vs. 66%) [5]. At the ASCO 2021 Annual Meeting, these promising data have now been reaffirmed. At 36 months, the PFS rate was 42% in the intervention arm versus 11% in the standard arm. The ORR advantage for the pembrolizumab group was even slightly more pronounced than in the previous analysis, at 45.1% vs. 33.1%. The time from randomization to the next line of therapy or patient death (PFS 2) was also significantly longer in the pembrolizumab group (54.0 vs. 24.9 months). In addition, pembrolizumab treatment showed a significantly higher OS rate at 36 months (61% vs. 50%). A cross-over is included in the study design, and the median OS for the pembrolizumab group had not been reached at the time of presentation at the 2021 ASCO Annual Meeting [6]. Pembrolizumab is approved for the treatment of metastatic CRC with MSI-H as first-line monotherapy in Switzerland [7].
CheckMate 142
The phase II CheckMate 142 trial is evaluating the role of nivolumab (trade name OPTIVO®; manufacturer BMS) after progression under/after, or intolerance to, fluorouracil in combination with oxaliplatin and/or irinotecan in patients with advanced or metastatic CRC MSI-H. 74 Patients with ≥3prior lines of treatment were included and received at least one dose of nivolumab 3 mg/kg body weight every two weeks. The primary endpoint here was ORR according to RECIST. After twelve months, the RR was 31% and 69% of the pa-tients had a stable disease course for at least twelve weeks. The median duration of treatment response had not been reached at the time of data inclusion [8]. The study design was expanded during the course and a combination immunotherapy with nivolumab and low-dose ipilimumab (trade name YERVOY®; manufacturer BMS) was investigated as the first line of treatment for the patient population with metastatic CRC. Here, a RR of 64%, a complete response (CR) in 9%, and sustained disease control at 24 months in 79% of cases were observed [9]. Nivolumab is approved as monotherapy or in combination with ipilimumab for the treatment of adult patients with metastatic CRC with MMR-d/MSI-H after prior fluoropyrimidine-based therapy in combination with irinotecan or oxaliplatin in Switzerland [7,10]. A recommendation for reimbursement of nivolumab by the Federal Office of Public Health (FOPH) of the Swiss Confederation is available, but not for ipilimumab [7].
Carcinomas of the esophagus, gastroesophageal junction and stomach
CheckMate 648
Until now, the prognosis of advanced squamous cell carcinoma of the esophagus has unfortunately been significantly limited with a median OS of approximately ten months – despite the use of chemotherapeutic agents. The CheckMate 648 trial is now comparing chemotherapy alone with the combination of chemotherapy and nivolumab, and with the combination of the first-line immunotherapeutics nivolumab and ipilimumab. Nearly 1000 patients with advanced squamous cell carcinoma of the esophagus were randomized to three treatment groups regardless of their PD-L1 status: (1) nivolumab (240 mg biweekly) and chemotherapy (fluorouracil and cisplatin four weeks); (2) nivolumab (3 mg/kg body weight biweekly) and ipilimumab (1 mg/kg body weight six weeks); (3) chemotherapy alone. The primary endpoints were OS and PFS in the patient group with PD-L1 expression ≥1%. Secondary endpoints were defined as OS, PFS, and RR in all patients regardless of PD-L1 expression.
At twelve months, the population with PD-L1 expression ≥1%showed an OS rate of 58% in the nivolumab + chemotherapy group vs. 37% in the chemotherapy group and a median survival benefit of 6.3 months for the nivolumab + chemotherapy combination. This was also confirmed in those patients who were included regardless of PD-L1 expression with an OS rate of 54% vs. 44% and a survival benefit of 2.5 months. However, when looking closely at the subgroup analysis with breakdown of survival benefit according to PD-L1 status, it is clear that there is a dominant effect of the PD-L1 positive subpopulation and that the detectable survival benefit is primarily driven by this subgroup. Whether PD-L1-negative patients benefit remains questionable. There were also clinically significant benefits for nivolumab + chemotherapy in terms of PFS and RR.
The comparison between nivolumab + ipilimumab and chemotherapy alone yielded similar results with an OS of 57% vs. 37% at 12 months and a survival benefit of 4.6 months with immunotherapy in the patient population with PD-L1 expression ≥1%. The analysis of subgroups according to PD-L1 status turns out very similar to the comparison of nivolumab + chemotherapy vs. chemotherapy. Based on these data, use of nivolumab + chemotherapy and nivolumab + ipilimumab in patients with advanced PD-L1-positive squamous cell carcinoma of the esophagus should be considered the new standard of care. Currently, nivolumab and ipilimumab are not yet approved for this indication in Switzerland [7,10]. In patients with PD-L1-negative esophageal cancer, chemotherapy alone remains an option.
Should nivolumab be approved in this indication in the first line of therapy, it would change the sequence of treatment options, at least for those patients with PD-L1 positive tumors. To date, nivolumab has been indicated for the treatment of advanced or recurrent adenocarcinoma of the stomach or GEJ after two or more prior systemic therapies – currently a cost approval is required in Switzerland [7,10]. Thus, if use is now shifted to the first line of treatment, this drug option must be reconsidered in later lines of treatment. Weighing patient-specific factors – for example, the presence of autoimmune diseases – could provide guidance in this regard. In principle, the use of the most effective therapeutic options at the beginning of treatment is a common oncologic principle and suggests the use of immunotherapy in the first line of treatment, unless contraindications exist.
CheckMate 649
Enzinger et al. established FOLFOX (fluorouracil, leucovorin, oxaliplatin) as a first-line chemotherapy regimen for the treatment of patients with metastatic carcinoma of the Ö/GEJ in 2016. Their study showed comparable OS and PFS with the then standard therapy ECF (epirubicin, cisplatin, fluorouracil) vs FOLFOX for this patient population, predominantly adenocarcinomas [11]. In both the intervention arm and the control group, chemotherapy had been combined with cetuximab. However, this does not correspond to any established standard therapeutic regimen in carcinomas of the Ö/GEJ and stomach and does not allow conclusions to be drawn about group-specific benefit when anti-EGFR-Ab is used in both study groups.
CheckMate 649 now compares standard chemotherapy alone (CAPOX, capecitabine, and oxali-platin tri-weekly or FOLFOX fluorouracil biweekly) with combination chemotherapy and nivolumab (360 mg tri-weekly or 240 mg biweekly) and with the immunotherapy combination of nivolumab + ipilimumab in the first-line setting. Patients with advanced adenocarcinomas of the Ö/GEJ and stomach were included. The primary endpoints for the nivolumab + chemotherapy group and for the chemotherapy group were PFS and OS in patients with a combined positive score (CPS) ≥5%– where CPS describes the proportion of PD-L1-positive tumor and immune cells (lymphocytes and macrophages) relative to all tumor cells. After an observation period of approximately one year, nivolumab + chemotherapy showed significant superiority in both PFS and OS. This effect was also verified for patients with a CPS ≥1%and all randomized patients [12]. Additional subgroup data have now been presented at the ASCO Annual Meeting. Here, the OS and PFS benefits of nivolumab + chemotherapy were more pronounced in patients with carcinomas with higher PD-L1 thresholds. In the overall population, a benefit for immunotherapy was seen regardless of PD-L1 status, although, as with CheckMate 648, this was driven by the PD-L1-positive subpopulation. Thus, for PD-L1-positive adenocarcinomas of the Ö/GEJ and stomach, FOLFOX combined with nivolumab is a new standard. Nivolumab is currently not approved for this indication in Switzerland [7]. Data on the nivolumab + ipilimumab study arm remain pending [13].
KEYNOTE-811
In 2008, the REAL-2 trial, a non-inferioritystudy, established CAPOX as an alternative to fluorouracil and cisplatin in the treatment of advanced carcinoma of the Ö/GEJ and stomach, regardless of histology. A fluoropyrimidine and a platinum compound were each combined with the anthracycline epirubicin [14].
The ToGA trial, a randomized phase III study, then evaluated the addition of trastuzumab, an anti-HER2-Ab, to this standard chemotherapy regimen (capecitabine and cisplatin, or fluorouracil and cisplatin) in the first-line setting. Patients with HER-2-positive advanced carcinomas of the stomach and GEJ were included. The triple combination with an anthracycline pre-existing from REAL-2 was not integrated in this study design due to the cardiotoxicity of this drug group and the cardiotoxic properties of trastuzumab. Due to significantly increased OS, the combination of chemotherapy and trastuzumab became the new standard of care in this patient population [15].
In the KEYNOTE-811 study, the addition of pembrolizumab 200 mg every three weeks to the above-mentioned standard of care in the first-line treatment is now being investigated for this same group of patients. Initial results were presented at the ASCO 2021 Annual Meeting: Significantly improved RR of 74.4% vs. 51.9% and CR rate of 11.3% vs. 3.1% were seen with pembrolizumab + chemotherapy. The rate of partial remission was 63% vs. 49%. The primary endpoints of OS and PFS are pending [16].
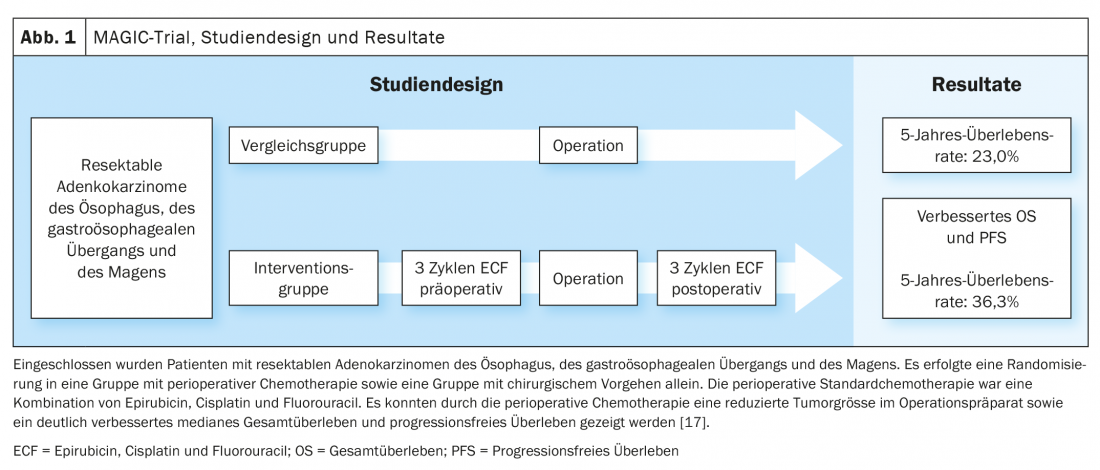
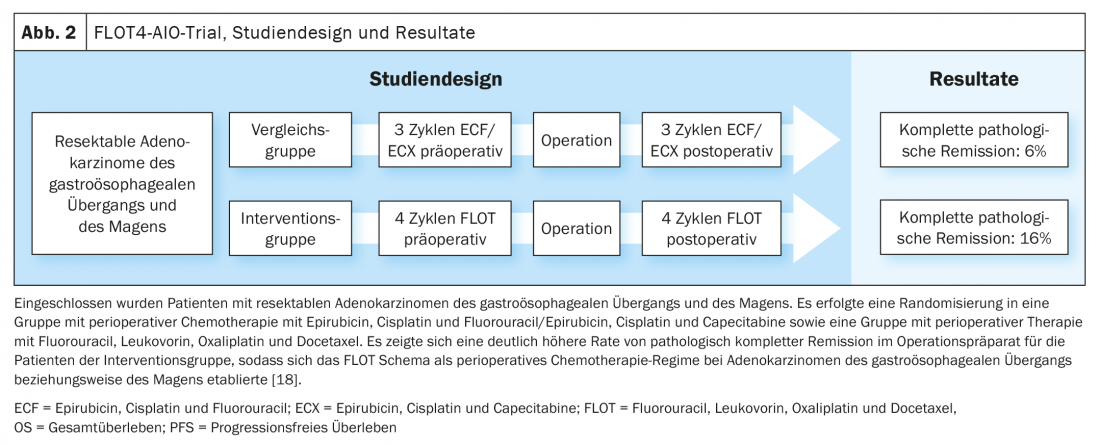
CROSS Update/Neo-AEGIS Update
Based on the data of the MAGIC trial and the further development of the treatment concept in the FLOT4-AIO trial, perioperative chemotherapy with FLOT (fluorouracil, leucovorin, oxaliplatin, docetaxel) became an established curative treatment option for gastroesophageal carcinomas (Fig. 1, Fig. 2) [17,18]. This contrasts with the trimodal therapy concept of the CROSS study (Fig. 3) [19]. The question of which of the two approaches – perioperative chemotherapy (MAGIC/FLOT protocol) or neoadjuvant radiochemotherapy analogous to CROSS – offers an advantage is currently being addressed by the Neo-AEGIS trial.
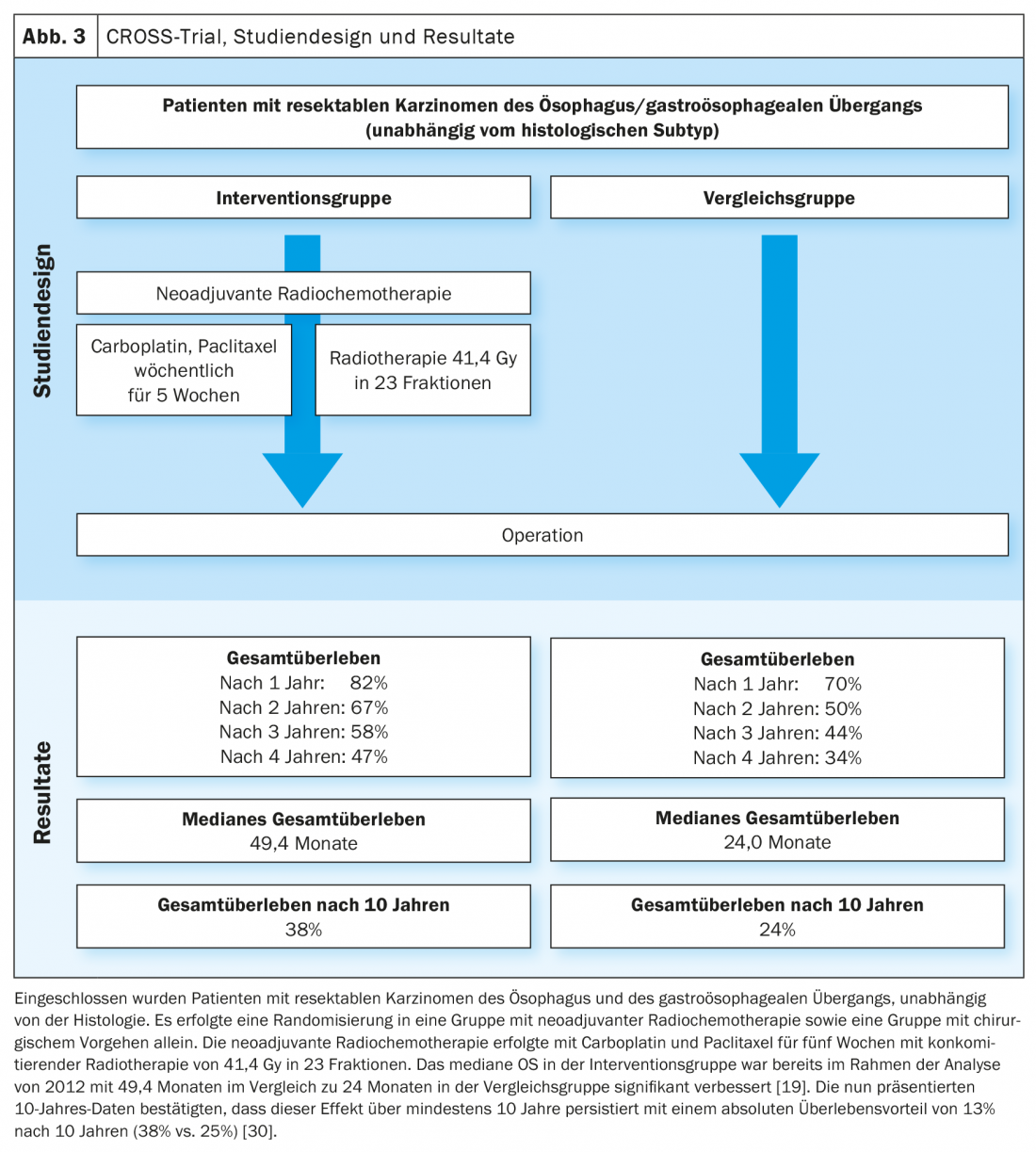
For this purpose, patients with resectable adenocarcinomas of the Ö/GEJ were randomized 1:1 to perioperative chemo-therapy (initially analogous to MAGIC, during the course analogous to FLOT4-AIO) and trimodal therapy analogous to CROSS [20]. In the current analysis (ASCO 2021), a non-inferiority approachwas used. There was no evidence of clear inferiority of perioperative chemotherapy compared with the trimodal approach. Nevertheless, patients treated analogously to CROSS showed a higher rate of resection in healthy (R0), more histologically negative lymph nodes after previous therapy (ypN0), a higher tumor regression grade and pathological CR. The rate of neutropenia ≥grade3 and the number of neutropenic sepses were higher in the perioperative chemotherapy group, whereas postoperative in-hospital death/postoperative pneumonia and ARDS and anastomotic insufficiency occurred at approximately the same rate. The primary endpoint of the study was OS, and this showed a comparable outcome after a three-year observation period [21].
Thus, a clear recommendation cannot yet be derived from the current data of the Neo-AEGIS study. Patient factors such as comorbidities should be considered when selecting a therapeutic approach. Furthermore, subsequent therapeutic options should also be included in the choice of therapy. Thus, adjuvant immunotherapy with nivolumab analogous to the CheckMate 577 trial is conceivable after the CROSS regimen. To investigate this, patients with evidence of residual carcinoma cells in the surgical specimen after trimodal therapy were randomized to an intervention group with nivolumab and a control group with placebo. There was significantly longer disease-free survival in the nivolumab group (22.4 months vs. 11.0 months) [22]. In this indication, nivolumab is approved in Switzerland, although a recommendation for cost coverage by the FOPH is currently not available [7,10].
GO2
Many studies that shape the oncology landscape are based on populations with a median age around 60 years. However, this only partially reflects the reality of everyday oncology practice, as the oncology patient population is increasingly composed of older patients with comorbidities or age-related limitations in health status.
This issue was addressed at the 2021 ASCO Annual Meeting for patients with advanced gastroesophageal cancer. The phase III GO2 trial included a patient population with a median age of 76 years. Palliative three-weekly chemotherapy with CAPOX was administered at three dosing levels: full dose (oxaliplatin 130 mg/m2 on day 1 and capecitabine 625 mg/m2 twice daily on days 1-14) and 80% and 60% of this dose. A non-inferiority approachshowed comparable PFS without significant reductions between the three dosing groups with better overall tolerability of the lowest dosing level [23].
Take-Home Messages
- Immunotherapy with pembrolizumab results in improved progression-free survival and overall survival and a higher treatment response rate than standard chemotherapy in the first-line treatment of advanced and metastatic colon cancer with high microsatellite instability (MSI-H).
- The combination of nivolumab and chemotherapy and combination immunotherapy with nivolumab and ipilimumab result in better overall survival than standard chemotherapy alone in the first-line treatment of advanced squamous cell carcinoma of the esophagus with PD-L1 expression ≥1%.
- The combination of nivolumab and chemotherapy results in better progression-free survival and overall survival than standard chemotherapy alone in the first-line treatment of advanced adenocarcinoma of the esophagus, gastroesophageal junction, and stomach, particularly in the PD-L1 positive subpopulation.
- Perioperative chemotherapy is noninferior to the trimodal treatment approach with neoadjuvant radiochemotherapy in the curative treatment of resectable adenocarcinomas of the esophagus and gastroesophageal junction in terms of overall survival, but more often results in neutropenia and achieves fewer R0 resections and
- Tumor regression in primary tumor and lymph nodes.
Literature:
- Siegel RL, et al: Cancer Statistics 2021. CA Cancer J Clin. 2021; 71: 7-33.
- National Cancer Registry, www.nkrs.ch/de/stat (last accessed September 2021).
- Battaglin F, et al: Microsatellite instability in colorectal cancer: overview of its clinical significance and novel perspectives. Clin Adv Hematol Oncol. 2018; 16(11): 735-745.
- Le DT, et al: PD-1 blockade in tumors with mismatch repair deficiency. N Engl J Med. 2015; 372(26): 2509-2520.
- André T, et al: Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020; 383(23): 2207-2218.
- André T, et al: Final overall survival for the phase III KN177 study: pembrolizumab versus chemotherapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC). Abstract 3500, ASCO 2021 Virtual Meeting, June 4-8, 2021.
- Federal Office of Public Health FOPH: List of specialties. www.spezialitätenliste.ch (last accessed September 2021)
- Overman MJ, et al: Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017; 18(9): 1182-1191. erratum in: Lancet Oncol. 2017; 18(9).
- Helwick C: CheckMate 142 Updated Analysis: First-Line Nivolumab Plus Low-Dose Ipilimumab in MSI-H/dMMR Metastatic Colorectal Cancer. ASCO Post. 2020. https://ascopost.com/issues/april-25-2020/checkmate-142-updated-analysis/
- Compendium OPTIVO. https://compendium.ch/product/1310903-opdivo-inf-konz-40-mg-4ml/mpro#MPro7100 (last accessed September 2021)
- Enzinger PC, et al: CALGB 80403 (Alliance)/E1206: A Randomized Phase II Study of Three Chemotherapy Regimens Plus Cetuximab in Metastatic Esophageal and Gastroesophageal Junction Cancers. J Clin Oncol. 2016; 34(23): 2736-2742.
- Janjigian YY, et al: First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastroesophageal junction, and esophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021; 398(10294): 27-40.
- Moehler MH, et al: First-line (1L) nivolumab (NIVO) plus chemotherapy (chemo) versus chemo in advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): Expanded efficacy and safety data from Checkmate 649. Abstract 4002, ASCO 2021 Virtual Meeting, June 4-8, 2021.
- Cunningham D, et al: Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008; 358: 36-46.
- Bang YJ, et al: Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet. 2010; 376: 687-697.
- Janjigian YY, et al: Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/GEJ) cancer: initial findings of the global phase 3 KEYNOTE-811 study. Abstract 4013, ASCO 2021 Virtual Meeting, June 4-8, 2021.
- Cunningham D, et al: Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006; 355: 11-20.
- Al-Batran SE, et al: Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): result from the phase 2 part of a multicentre, open-label, randomized phase 2/3 trial. Lancet Oncol. 2016; 17: 1697-1708.
- Van Hagen P, et al: Peroperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366; 2074-2084.
- Reynolds JV, et al: ICORG 10-14: NEOadjuvant trial in Adenocarcinoma of the oesophagus and oesophagGastric junction International Study (Neo-AEGIS). BMC Cancer. 2017; 17: 401.
- Reynolds JV, et al: Neo-AEGIS (Neoadjuvant trial in Adenocarcinoma of the Esophagus and Esophago-Gastric Junction International Study): Preliminary results of phase III RCT of CROSS versus perioperative chemotherapy (Modified MAGIC or FLOT protocol). Abstract 4004, ASCO 2021 Virtual Meeting, June 4-8, 2021.
- Kelly RJ, et al: CheckMate 577 Investigators. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021; 384(13): 1191-1203.
- Hall PS, et al: Efficacy of Reduced-Intensity Chemotherapy with Oxaliplatin and Capecitabine on Quality of Life and Cancer Control Among Older and Frail Patients With Advanced Gastroesophageal Cancer. The GO2 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021; 7(6): 869-877.
- De Gramont A, et al: Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000; 18(16): 2938-2947.
- Douillard JY, et al: Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000; 355(9209): 1041-1047. erratum in: Lancet. 2000; 355(9212): 1372.
- Saltz LB, et al: Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008; 26(12): 2013-2019. erratum in: J Clin Oncol. 2008; 26(18): 3110. erratum in: J Clin Oncol. 2009; 27(4): 653.
- Douillard JY, et al: Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013; 369(11): 1023-1034.
- Van Cutsem E, et al: Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015; 33(7): 692-700.
- Cremolini C, et al: FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015; 16(13): 1306-1315.
- Eyck BM, et al: Ten-Year Outcome of Neoadjuvant Chemoradiotherapy Plus Surgery for Esophageal Cancer: The Randomized Controlled CROSS Trial. J Clin Oncol. 2021; 39(18): 1995-2004.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(5): 12-18.