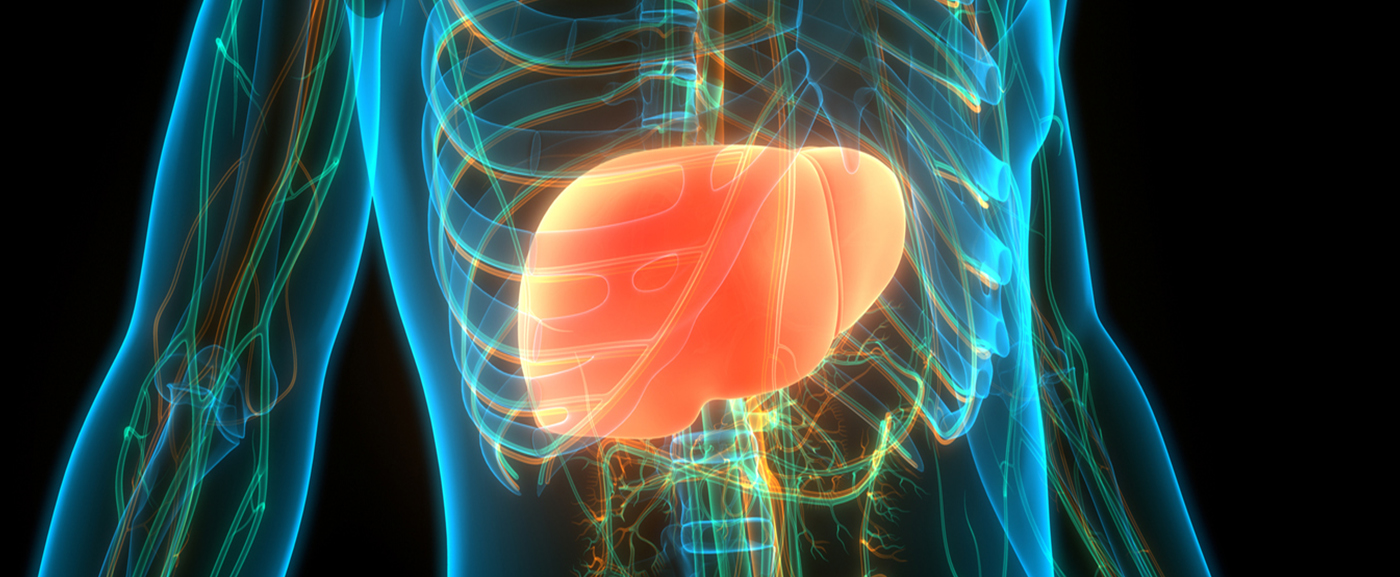
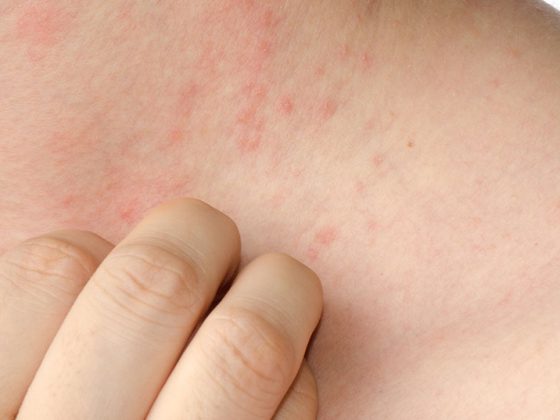
In terms of tolerability, ozanimod compares favorably with other sphingosine-1-phosphate receptor modulators [1–3]*. In the pivotal studies, drop-out rates due to adverse events were approximately 3%, with comparable rates in each of the different treatment groups. The adverse drug reactions most commonly observed during ozanimod therapy were nasopharyngitis, upper respiratory tract infections, urinary tract infections, elevated alanine aminotransferase and γGT levels, hypertension, and headache.
In both phase III trials conducted, the most common reasons given for discontinuation were “voluntary withdrawal ” and side effects [2,3]. Study discontinuations directly related to adverse drug events occurred in 1.5% and 2.9%, respectively, in the ozanimod therapy groups and in 3.6% in the interferon therapy group in the SUNBEAM study with a minimum duration of 12 months (Table 1). In the RADIANCE trial with an intervention duration of 24 months, drop-outs directly attributable to adverse events were estimated to be about 3% in the intervention groups and 4.1% in the control group. Thus, therapy with interferon β1a resulted in the greatest number of clearly side-effect-associated treatment discontinuations in both cohorts, although the tolerability of the new agent appears to be good [2,3].

Nasopharyngitis as the most common side effect
Patients who experienced adverse drug reactions during pivotal trials most commonly dealt with nasopharyngitis [2,3]. Other infectious diseases such as upper respiratory tract infections, urinary tract infections or isolated pharygitis occurred somewhat less frequently with mostly mild courses. Elevations in liver enzymes, particularly ALT and γGT, were observed significantly more frequently with ozanimod therapy than with interferon β1a treatment in both phase III studies. Therefore, caution is advised in patients with liver failure and the drug is contraindicated in individuals with Child- Pugh class C [4]. Other side effects included hypertension, which increased especially in the RADIANCE study, hypercholesterolemia, headache, back pain, arthralgias, fatigue, and upper abdominal pain (Overview 1). Under close ophthalmologic monitoring, rare cases of macular edema occurred in both phase III trials [2,3]. While a total of 5 patients were affected in the RADIANCE cohort, 2 of whom received interferon β1a, exactly one participant per study arm developed the condition in the SUNBEAM study. In all cases, risk factors or causative comorbidities such as choroidopathies existed before therapy. In each case, treatment was discontinued immediately after diagnosis.
To date, the frequencies of adverse drug reactions are consistent with those observed in the two active-controlled clinical trials [5]. Nevertheless, due to the relatively recent introduction of the drug, close further monitoring, as conducted by the current open-label study DAYBREAK [5] and, of course, the drug regulatory authorities, is important. The bottom line is that infections are the most common side effects, which may be explained considering the mode of action with consecutive downregulation of the immune system.
Cost-benefit calculation in comparison
So far, only an indirect comparison by Swallow E et al. exists, which compares ozanimod with similar drugs such as the S1P receptor modulator fingolimod [1]*. This is favorable not only for the safety profile, but also with respect to the tolerability and thus the cost-benefit profile of the new agent.
After one year of therapy, there were significantly fewer adverse drug reactions and liver enzyme elevations, as well as greater mean lymphocyte counts under treatment with Ozanimod [1]. Fewer side effects were also observed with Ozanimod therapy when comparing agents at 2 years. In particular, liver infections, liver elevations, and bradycardia occurred less frequently during treatment with ozanimod. The spectrum of adverse drug reactions was comparable for both drugs. In the pivotal studies on fingolimod, there were significantly more study discontinuations due to side effects overall, supporting the thesis of comparatively better tolerability of ozanimod.
A direct comparison with alternative agents, also with regard to drug tolerance, is currently still pending and long-term data must be awaited. However, ozanimod appears to be a well-tolerated agent, as indicated by the data to date.

* which occurred in >2% of patients on ozanimod therapy with an incidence at least 1% higher than in the interferon group OZA=ozanimod; IFN=interferon beta-1a
Literature