In a phase III trial, pembrolizumab improved overall survival in patients with recurrent or metastatic head and neck squamous cell carcinoma.
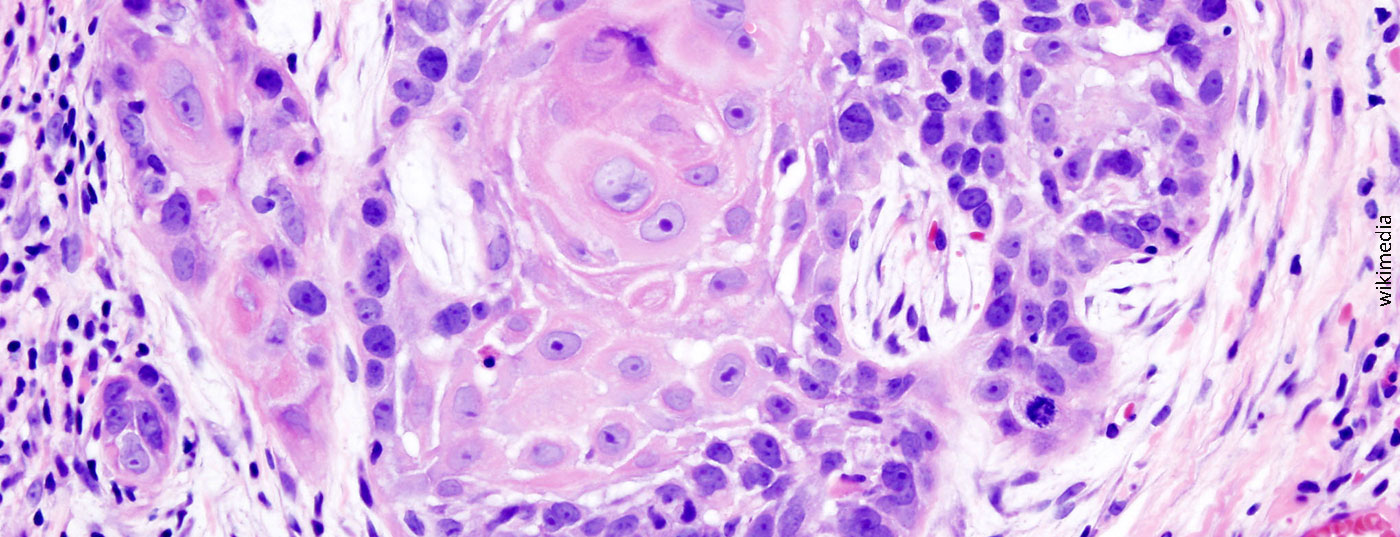
The phase III KEYNOTE-048 trial presented at the ASCO 2019 annual meeting evaluated the PD-1 (programmed cell death 1 protein) inhibitor pembrolizumab (KEYTRUDA®) both as monotherapy and in combination with chemotherapy for first-line treatment of recurrent or metastatic head and neck squamous cell carcinoma (HNSCC). This is the first publication of data from this study on overall survival with pembrolizumab in combination with chemotherapy as a function of tumor PD-L1 expression and from the pembrolizumab monotherapy arm in the overall collective and independent of tumor PD-L1 status. The interim analysis was presented at the ESMO 2018 Annual Meeting. This showed superior overall survival with pembrolizumab in combination with chemotherapy in the overall population as well as with pembrolizumab monotherapy in patients whose tumors expressed PD-L1 with a combined positive score (CPS) ≥20 and ≥1 compared to patients on the current standard of care (EXTREME regimen). Squamous cell carcinomas are classified according to the currently valid TNM systems of the Union internationale contre le cancer (UICC) or the American Joint Committee on Cancer (AJCC). According to the AJCC, squamous cell carcinomas of the neck-head region are distinguished from those of other localizations.
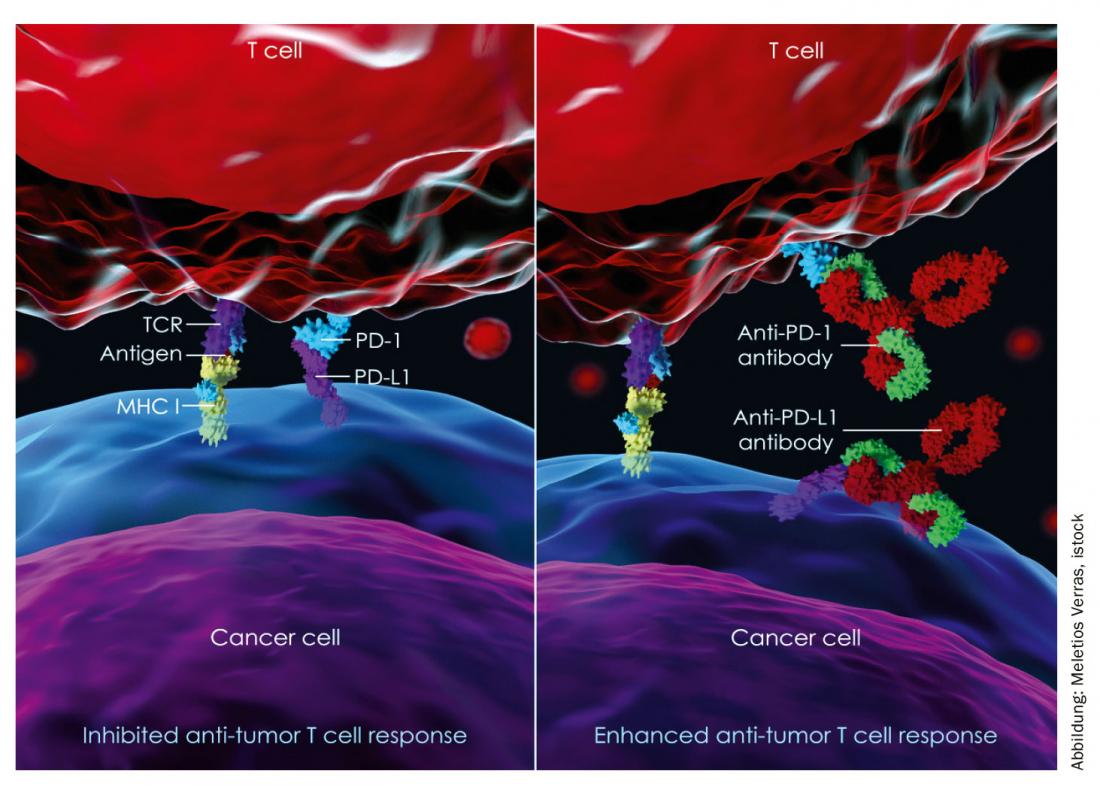
Source: MSD
DERMATOLOGIE PRAXIS 2019; 29(5): 48