T1-T2 N0 tumors undergo primary surgery. Proximal esophageal cancer is an indication for radio-chemotherapy alone (RCT). In contrast, T3-T4 and N+ carcinomas are treated trimodally when possible. Neoadjuvant RCT increases resection rates and improves disease-free and overall survival. In cases of inoperability, definitive RCT can even achieve five-year survival of up to 30%. Trimodal therapy for esophageal cancer should be performed at an experienced center. Perioperative chemotherapy alone improves the results of surgery for gastric cancer. In an N+ situation, combined adjuvant RCT should be considered.
Charles Moertel first reported simultaneous radio-chemotherapy (RCT) for unresectable gastrointestinal tumors in 1969 in the Lancet [1]. Today, the combination of the two therapeutic modalities, along with surgery, is standard practice for many tumors from the esophagus to the anal canal. With the development of fiberoptic endoscopy, perfection of imaging techniques, and standardization of staging, the tumor-specific risk for individual patients can be better assessed. Technological advances in radiotherapy have refined the optimization of dose application to the tumor while sparing the healthy surrounding organs at risk as much as possible.
Despite the introduction of new agents in various combinations, the development of different EGFR antibodies, and the discovery of important biomarkers such as KRAS, NRAS, and BRAF, 5-fluouracil, which has been used for decades, is still an important component of today’s polychemotherapy. Overall, this has resulted in a slight increase in five-year survival rates over the past 30 years for both esophageal and gastric cancer.
Five randomized trials of the use of preoperative radiotherapy alone have provided no benefit in terms of survival. Likewise, three randomized trials showed improvement in local control but no survival benefit with postoperative radiotherapy alone.
Biological features of esophageal carcinoma.
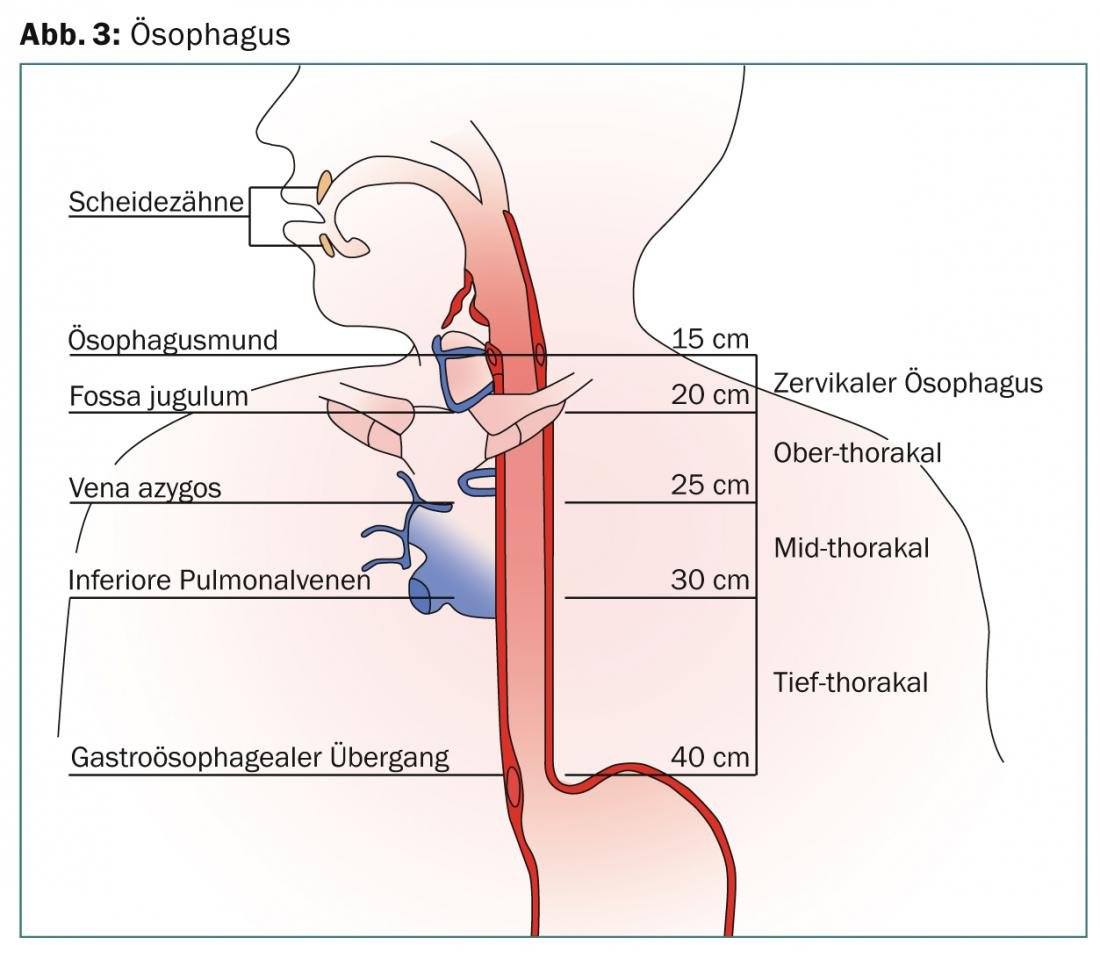
The esophagus extends 25 cm from the cricopharyngeus muscle cranially to the esophagogastric junction caudally. The endoscopically visible Z-line defines the transition from pavement cell to cylinder epithelium. In Barrett’s esophagus, the pavement epithelium is replaced by highly stratified cylindrical epithelium. According to the latest AJCC report, the organ is divided into the cervical, upper thoracic, mid-thoracic, and deep thoracic regions (Fig. 1) .

The length is calculated endoscopically from the incisors to the entrance of the stomach in centimeters. The multi-layered wall structure of the organ is traversed by an abundant network of lymphatic vessels. Lymph node metastases in the truncus coeliacus, previously scored as M1, are defined as M0 according to the latest AJCC classification (2010), as they are covered in the irradiation volume by default. Lymphogenic metastasis correlates with T stage, tumor length, and degree of differentiation and can be as high as 38-60% in a T2 lesion, with microscopic involvement in >60% of cases, which cannot be detected with current imaging techniques. Lymph node involvement depends on the primary tumor location (Fig. 2).
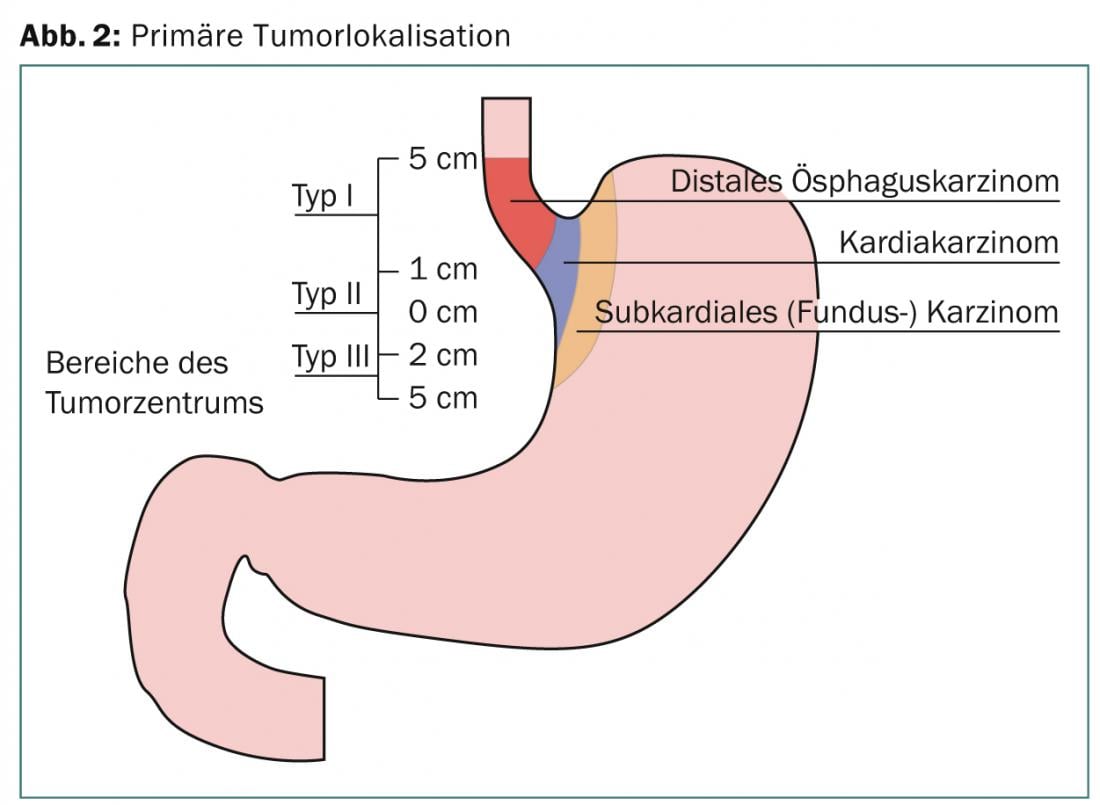
At diagnosis, approximately half of esophageal tumors are primarily unresectable or have distant metastases. With rare exceptions, these are usually adenocarcinomas or pavement cell carcinomas, with the first type predominating and clearly increasing in incidence in recent years. These are two distinct tumor entities with different etiology, epidemiology, and prognosis [2]. This fact is also taken into account in the 7th edition of AJCC-Staging-Manuel [3]. The tumor localization at the gastroesophageal junction is also defined here. These include tumors of the distal esophagus extending up to 5 cm into the stomach (Sievert III) (Fig. 3). In most prospective studies, the different tumor entities are not always distinguished separately. In a large proportion of distal tumor localizations, tumors of the gastroesophageal junction are included.
Staging
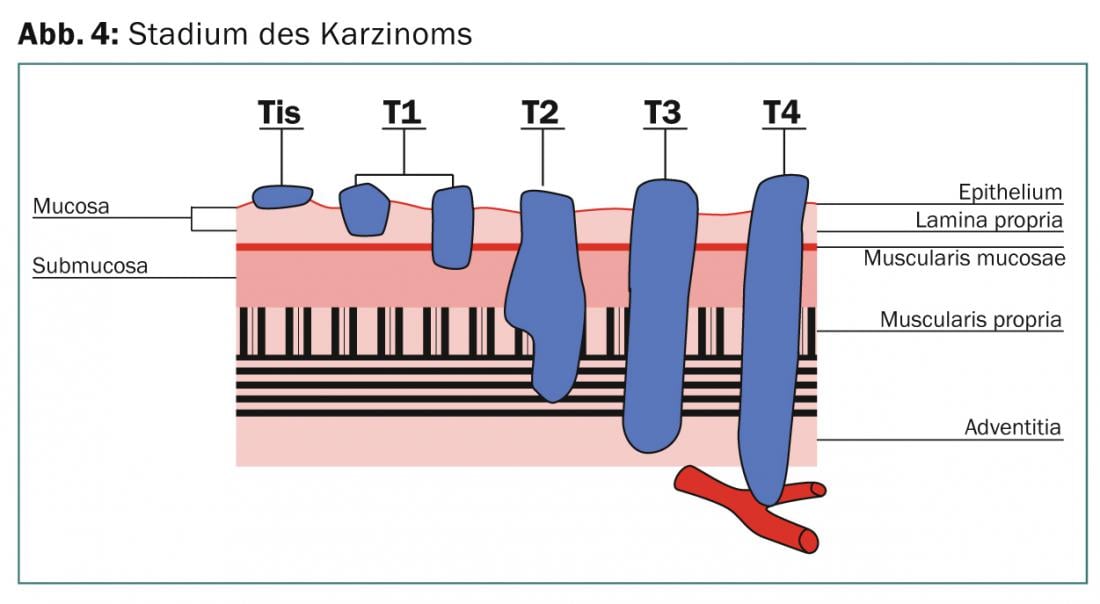
Diagnosis is made by endoscopy and biopsy sampling. A CT of the thorax and abdomen is performed in all patients, and endoluminal sonography determines the T and N stages. Before deciding on definitive RCT or trimodal treatment, PET-CT is performed as a standard procedure because distant metastases are found in up to 22% of cases during this clarification examination [4]. If the tumor is located above the carina, an esophagobronchial fistula should be excluded by bronchoscopy if there is clinical suspicion (Fig. 4).
If the tumor is distal, laparoscopy should be performed to exclude peritoneal metastasis before trimodal therapy.
The neoadjuvant RCT before planned surgery.
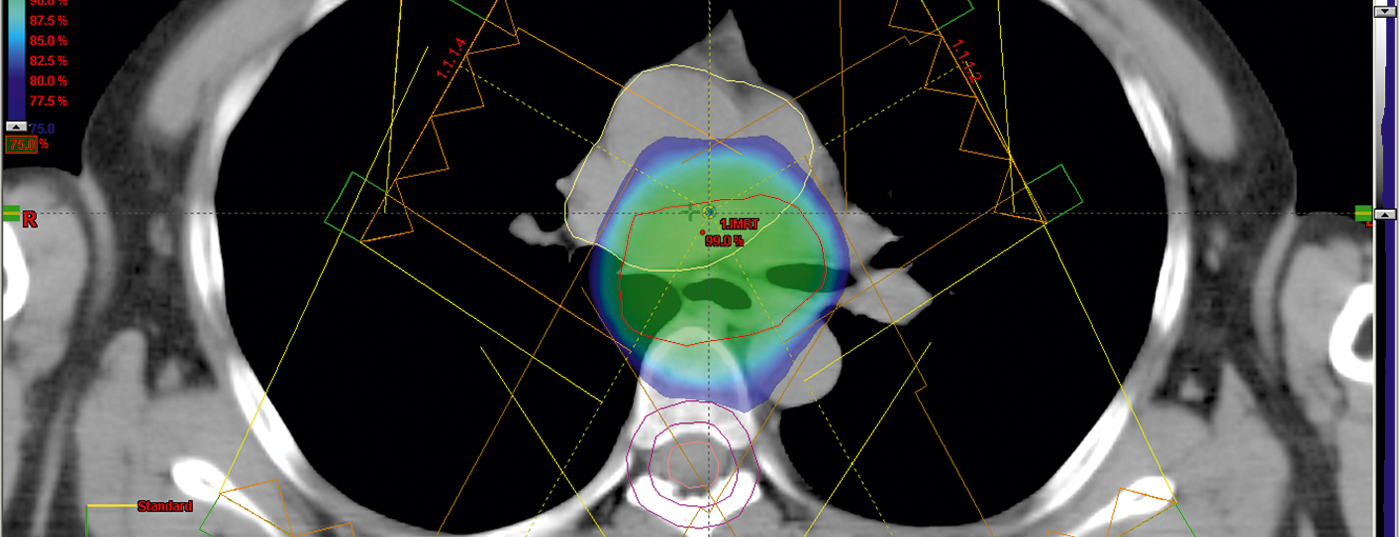
Treatment of esophageal cancer with curative intention is nowadays offered at a center hospital with an experienced interdisciplinary team. CT-guided 3-D radiation planning is performed first, using a highly specialized technique that includes intensity-modulated dose application and an image-guided (Image-Guided RT) radiation sequence.
In the neoadjuvant setting, 45 grays (Gy) are usually administered over five weeks. In the 1990s, simultaneous chemotherapy according to the so-called Herskovic regimen (combination of cisplatin and 5-fluouracil) was administered to patients as standard [5]. The RTOG-8501 trial was the first to show that simultaneous radio/chemotherapy was significantly superior to radiotherapy alone [6].
Neoadjuvant RCT improves survival and is now standard of care for tumors >T2 and/or N+. Achieving R0 resection is one of the most important goals to ensure longer-term tumor-free survival.
In case of impossibility of food intake, a jejunostomy should be created instead of a PEG in case of planned surgery in order not to impair the later formation of a “neo-esophagus” after resection.
Proximal esophageal carcinomas are generally treated with definitive RCT.
The PET-CT result is relevant for therapy planning; the PET-avid part correlates very well with the endoscopic result of tumor extension [7]. Histologic studies after esophagectomy have shown that marging 3 cm proximal and distal to the tumor can cover 94-100% of microscopic subclinical involvement [8]. As a rule, cranio caudal 3-5 cm and lateral 2 cm to the GTV (macroscopic tumor volume) are now defined as safety margins. Proximal tumor location includes the supraclavicular lymph node stations and distal location includes the coeliac lymph node stations [8]. The distal GEJ tumors may require those of the small and large curvature of the stomach in addition to inclusion of the coeliac lymph nodes.
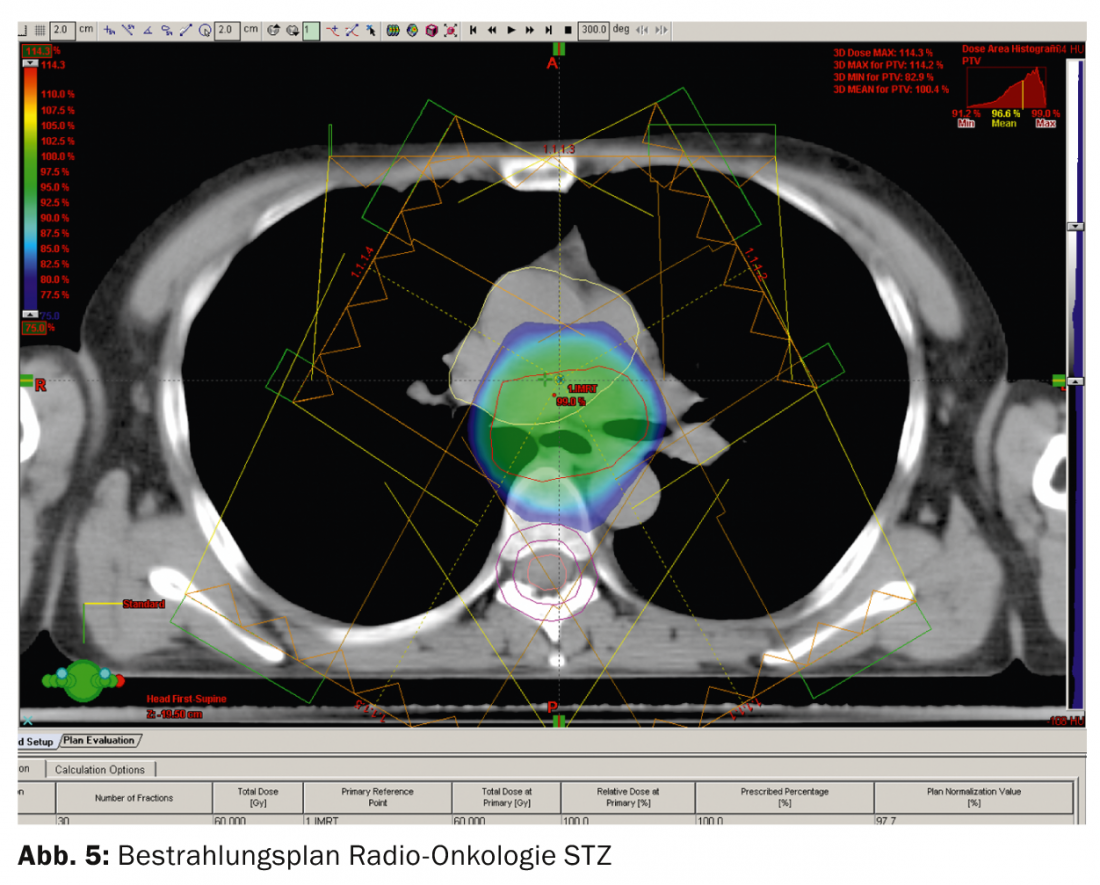
Optimal multi-field irradiation allows the permissible doses to healthy organs (lungs, heart, liver) or constraints to be met (Fig. 5).
Surgery is usually performed six to eight weeks after completion of simultaneous RCT.
Walsh [9] showed a significant advantage in favor of bimodal therapy in 110 patients with adenocarcinomas in combination with 40 Gy and cisplatin/5-FU compared with surgery alone. However, the work was criticized for poor surgical results. Three randomized trials [10–12] showed a survival benefit in favor of combined therapy and a trend toward significantly better outcomes when complete tumor remission was achieved. However, patient numbers in the trials were modest. The largest study in terms of numbers, the so-called Cross Trial, was published by van Hagen in the New England Journal of Medicine in 2012. In the study, comparing neoadjuvant RCT with taclitaxel and cisplatin with 41.4 Gy in 23 fractions vs surgery alone, 366 patients were randomized in each of the two arms; 75% had adenocarcinoma and 23% had pavement cell carcinoma. R0 resection was 92 vs. 69% in the combined group with surgery alone. A 29 percent pathologic complete remission was achieved. Three-year results showed a significant improvement in survival of 58 vs. 44% with surgery alone. Since then, this regimen has been accepted as the standard for resectable esophageal and gastroesophageal tumors. A meta-analysis in the 2011 Lancet Oncology [13] confirms the advantage of neoadjuvant therapy, resulting in a nonsignificant advantage in favor of RCT vs. chemotherapy. The absolute survival benefit at two years was 8.7%, and this was independent of histology. For Siewert I + II gastroesophageal junction tumors, preference was given to neoadjuvant RCT two years ago at the first GI-EORTC conference [14].
Neoadjuvant chemotherapy for esophageal cancer.
The Medical Research Council (MRC) group randomly assigned 800 patients with patch cell and adenocarcinoma of the esophagus to a two-cycle cisplatin/5-FU group compared with radiotherapy alone. Even at six years, patients show a significant survival benefit of 23 vs. 17% regardless of histology [15].
The so-called Magic Trial with use of perioperative chemotherapy with epirubicin, cisplatin, and 5-FU (ECF) compared with surgery alone shows a five-year survival of 36 vs. 23% and is now preferred as neoadjuvant therapy for resectable gastric cancer. Each group randomized 250 patients with GEJ or gastric cancer.
Definitive RCT in esophageal cancer.
There are no randomized trials directly comparing surgery and radiation. Radiotherapy alone results in a median survival of 6-12 months and a five-year survival of <10%. Radiotherapy alone, either percutaneous or with intraluminal brachytherapy, is therefore used only in the palliative situation.
Long-term data from the RTOG 85-01 trial [16] show a five-year survival of 26% in favor of RCT. Overall, they show significant improvement in local control, median and overall survival. These data are quite comparable to those with surgery alone in the MRC trial with a five-year survival of approximately 20%. In general, the value of subsequent surgical intervention does not appear to be significantly determinant of overall survival despite improvement in local control. A randomized trial of 445 patients by the Frenchman Bedenne [17] concludes that if the response rate is good after simultaneous RCT, subsequent surgery does not improve survival. In a similarly designed study by Stahl [18], although local tumor control was improved by subsequent surgery, three-year survival was not significantly different. After induction chemotherapy with 5-FU, leucovorin, etoposide, and cisplatin followed by 40 Gy of radiotherapy, patients were randomized to one group with direct surgery and one with continuation of RCT up to 60 Gy. Those patients who responded to induction chemotherapy benefited from subsequent surgery in terms of survival at three years, but mortality was significantly higher in the operated group. The authors found that surgery rate improved local control but not overall survival. Further studies need to determine whether and in which patients surgery can be omitted after RCT.
Since both therapeutic modalities including surgery have a high locoregional recurrence rate, several trials are underway testing new agents such as taxanes, gemzitabine, or EGFR receptor inhibitors.
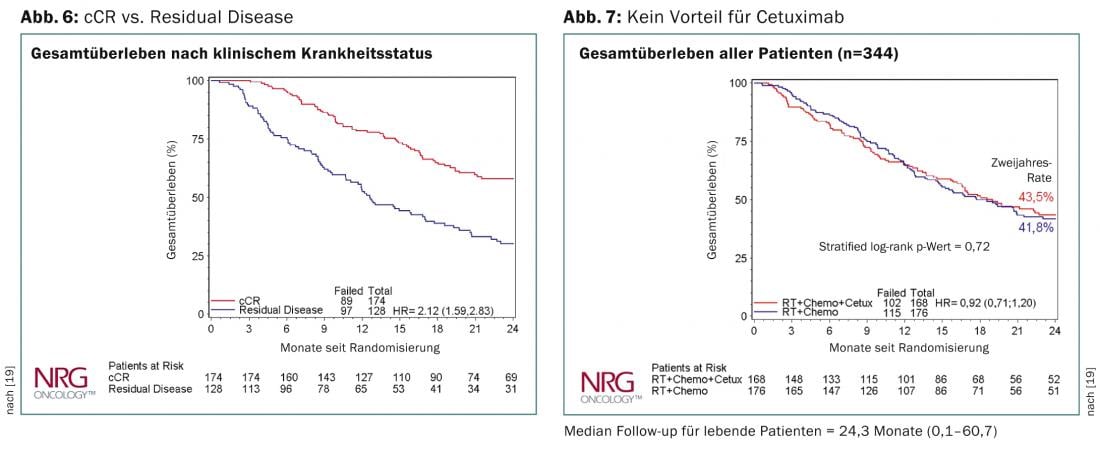
Data from the RTOG-436 trial were presented at this year’s ASCO meeting in Chicago [19]. This is a randomized phase 3 trial to assess the effect of adding cetuximab to definitive RCT with paclitaxel and cisplatin. 344 patients were randomized overall, and the clinically complete remission rate was 70%. The proportion of T3/T4 tumors was reported to be 80%, that of N+ 66%, and two-thirds of patients had adenocarcinoma. Despite the addition of cetuximab, overall survival was not significantly different between the two arms. There was also no difference in the result regarding histology. Patients with a clinically complete remission had a significantly better overall survival after two years (Fig. 6 and 7). The SCOPE study [20] comes to the same conclusion and cannot recommend the addition of cetuximab in the definitive RCT.
SAKK-75-02 successfully tested the feasibility of induction chemotherapy with docetaxel and cisplatin followed by combined RCT with 45 Gy and the same agents. This showed a higher response rate for the patch cell carcinomas. In the subsequent SAKK75-06, the addition of Erbitux showed a promising response rate. The following prospective randomized phase III trial (SAKK 75-08) is testing the value of multimodal therapy with or without cetuximab. The study was recently closed after accrual from >300 patients.
Preoperative RCT vs. preoperative chemotherapy.
A direct comparison of preoperative RCT with neoadjuvant chemotherapy alone was made in a German study [21]. Unfortunately, due to bad accrual it had to be closed early. The study authors find a better pathologic complete remission of 2 vs. 16% as well as improved local control of 59 vs. 76% and improved three-year survival 28 vs. 47% in favor of the combined modality.
Adjuvant RCT in gastric cancer.
In the 2001 study published by Macdonald in the Journal Clinical Oncology, 20% of patients are listed as having a GEJ. In these, adjuvant RCT produced a significant benefit for median survival of 36 vs. 27 months with surgery alone [22]. Long-term follow-up shows a further benefit for GEJ tumors in terms of overall survival and tumor freedom [23].
At the 2014 ASCO meeting in Chicago, the ARTIST study was presented. It is a randomized phase 3 trial comparing adjuvant chemotherapy alone with capecitabine/cisplatin (XB) vs. XB plus simultaneous radiotherapy with capecitabine [24]. It is a work from South Korea with a D-2 resection of gastric carcinoma. A total of 448 patients were randomized. No differences in disease-free and overall survival were found in either arm.
In contrast, patients with positive lymph node metastases showed a statically significant improvement in disease-free survival at three years of 76 vs. 72% in favor of radiotherapy.
Norbert Lombriser, M.D.
Literature:
- Moertel CG, et al: The Lancet 1969 Oct 25; 2(7626): 865-867.
- Siewert A: Surg 2001; 234-360.
- Rice TW, Blackstone EH, Rusch VW: Rice Annals of Surgical Oncology 2010; 17(7): 1721-1724.
- Flammen P, et al: JCO 2000; 18: 3202.
- Al-Sarraf M, et al: JCO 1997; 15(1): 277-284.
- Herskovic A, et al: NEJM 1992; 326(24): 1593-1598.
- Konski A, et al. Int J Radiat Oncol Biol Phys 2005; 61: 1123-1128.
- Hazard L, et al: J Natl Compr Canc Netw 2008; 6: 870-878.
- Walsh TN, et al: N Engl J Med 1996; 335: 462-467.
- Urba SG, et al. : J Clin Oncol 2001; 19: 305-313.
- Bosset JF, et al: N Engl J Med 1997; 337: 161-167.
- Burmeister BH, et al: Lancet Oncol 2005; 6: 659-668.
- Sjoquist KM, et al: Lancet Oncology July 2011; 12(7): 681-692.
- Lutz MP: European Journal of Cancer 2012; 48: 2941-2953.
- Allum WH, et al: J Clin Oncol 2009; 27: 5062-5067.
- Cooper JS, et al: Radiation Therapy Oncology Group. JAMA 1999; 281: 1623-1627.
- Bedenne L, et al: J Clin Oncol 2007; 25: 1160-1168.
- Stahl M, et al: J Clin Oncol 2005; 23: 2310-2317.
- ASCO Meeting 2014 Abstract 4007.
- Crosby T, Hurt CN: Lancet Oncology 2013; 14(7): 627-637.
- Stahl M, et al: J Clin Oncol 2009; 27: 851-856.
- Macdonald JS, et al: N Engl J Med 2001; 345: 725-730.
- Smalley SR, et al: J Clin Oncol 2012; 30(19): 2327-2333.
- ASCO Meeting 2014 Abstract 4008.
- Cunningham D, et al: N Engl J Med 2006; 355: 11-20.
- van Hagen P, et al: N Engl J Med 2012; 366: 2074-2084.
- Ruhstaller T: Annals of Oncology 2009; 20: 1522-1528.
InFo Oncology & Hematology 2014; 2(6): 6-10.