Interventional clinical trials can be divided into phase I, II and III. So far, so good. But what does that actually mean exactly? Here’s a quick refresher for those who are not directly involved in clinical research and whose study time was a bit longer ago.
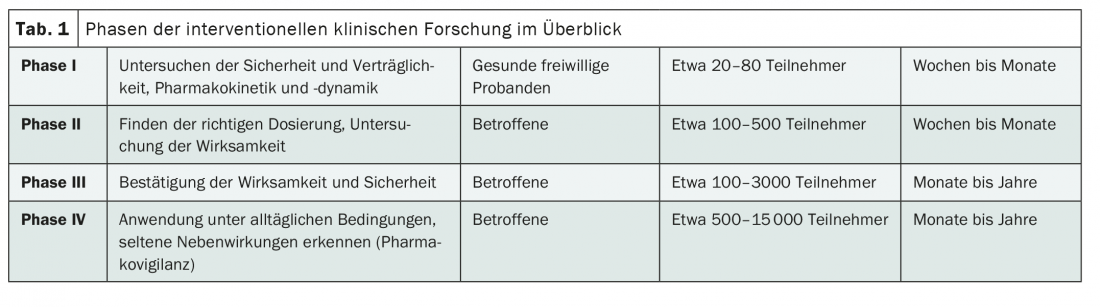
Each intervention study – that is, any study in which participants receive an active form of treatment – can be assigned to a specific phase of development of the intervention being tested, for example, the drug being tested (Tab.1). In particular, the aim is to protect study participants from potential adverse effects and to allow the intervention to be characterized as accurately as possible in different subjects. By the way, this has nothing to do with randomization; not every Phase III study is automatically a controlled randomized trial.
Step by step from I to III
Classically, the preclinical testing of an intervention is followed – as the name suggests – by a Phase I trial. The intervention or drug is tested in humans for the first time. The focus here is on testing compatibility [1]. Usually, a Phase I study involves a few dozen healthy volunteers. Pharmacokinetics and pharmacodynamics at different dosages are studied in detail. In this way, initial findings on side effects in humans and suitable dosages will be collected [1].
Only in the second phase is the focus on effectiveness. This involves people with the disease for the first time, which makes it essential for pharmaceutical companies and clinical research organizations to collaborate with hospitals. Usually, about 100 to 500 patients are included – with or without a control group, with or without blinding [1].
In much larger clinical trials, the Phase III trials, the intervention is then ideally tested on several thousand patients. It will be tested whether efficacy and tolerability can be reproduced in a large study population. Subgroup analyses are also important here, as they can provide information about which patients benefit particularly – and which tend to benefit less – from the intervention. Phase III trials are often comparative studies with existing therapies and/or placebo [1]. These are also the most meaningful. The more advanced the development of an intervention and the larger the study, the more important it is to plan well in advance. Endpoints must be determined and the statistical analysis established as accurately as possible. This is because, for example, it is usually not possible to catch up on a data collection of more than 1000 patients.
Depending on the intervention and disease, Phase III trials can be difficult to conduct. This is the case, among others, for very rare diseases. There are also cases in which randomized-controlled phase III trials are hardly ethically justifiable – for example, when high clinical efficacy is already apparent in the 2nd phase. Due to this problem, drugs are repeatedly approved before the corresponding phase III studies have been completed, which is currently a hot topic in oncology. Often, inclusion in a clinical trial is what allows access to an (unapproved) drug in the first place. On the one hand, this can save lives, but it is also a lottery – and a reason to refer appropriate patients to specialized centers that participate in clinical trials. Overall, the road from Phase I study to approval is long and has a meager chance of success of just under 10%. And this halves to 5.1% in the development of cancer drugs. Most trials are terminated in phase II [2].
Not to be forgotten: Phase 0 and IV
Although clinical development usually focuses on Phase I-III, Phase 0 and IV trials also play an important role in medical innovation. While Phase 0 studies involve the testing of human material, Phase IV studies take place after market approval. Accordingly, the latter are limited to approved indications, dosages and forms of administration. The size of the population allows, among other things, the detection of rare side effects [3].
Literature:
- Clinical Phase. www.interpharma.ch/themen/fuhrend-in-forschung-entwicklung/der-weg-eines-medikaments/klinische-phase-phase-i-ii-iii (last accessed 11/21/2021).
- Thomas DW, et al.: Clinical Development Success Rates 2006-2015. www.bio.org/sites/default/files/legacy/bioorg/docs/Clinical%20Development%20Success%20Rates%202006-2015%20-%20BIO,%20Biomedtracker,%20Amplion%202016.pdf (letzter Zugriff am 21.11.2021).
- Study Phases. www.dimdi.de/dynamic/de/glossar/glossareintrag/Studienphasen (last accessed 11/21/2021).
- What are the types of clinical trials? www.viomedo.de/posts/welche-arten-von-klinischen-studien-gibt-es (last accessed 11/21/2021).
InFo ONCOLOGY & HEMATOLOGY 2021; 9(6): 42.











